The RNA helicase Dhh1p cooperates with Rbp1p to promote porin mRNA decay via its non-conserved C-terminal domain
- PMID: 21998293
- PMCID: PMC3273804
- DOI: 10.1093/nar/gkr803
The RNA helicase Dhh1p cooperates with Rbp1p to promote porin mRNA decay via its non-conserved C-terminal domain
Abstract
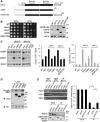
The yeast RNA helicase Dhh1p has been shown to associate with components of mRNA decay and is involved in mRNA decapping and degradation. An RNA-binding protein, Rbp1p, is known to bind to the 3'-UTR of porin (POR1) mRNA, and induces mRNA decay by an uncharacterized mechanism. Here, we show that Dhh1p can associate with POR1 mRNA and specifically promote POR1 mRNA decay via its interaction with Rbp1p. As compared to its mammalian homolog RCK/p54/DDX6, Dhh1p has a unique and long extension at its C-terminus. Interestingly, this non-conserved C-terminal region of Dhh1p is required for interaction with Rbp1p and modulating Rbp1p-mediated POR1 mRNA decay. Notably, expression of a C-terminal 81-residue deleted Dhh1p can fully complement the growth defect of a dhh1Δ strain and retains its function in regulating the mRNA level of an RNA-binding protein Edc1p. Moreover, mammalian DDX6 became capable of interacting with Rbp1p and could confer Rbp1p-mediated POR1 mRNA decay in the dhh1Δ strain upon fusion to the C-terminal unique region of Dhh1p. Thus, we propose that the non-conserved C-terminus of Dhh1p plays a role in defining specific interactions with mRNA regulatory factors that promote distinct mRNA decay.
Figures




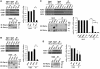
References
-
- Wilusz CJ, Wilusz J. Bringing the role of mRNA decay in the control of gene expression into focus. Trends Genet. 2004;20:491–497. - PubMed
-
- Parker R, Song H. The enzymes and control of eukaryotic mRNA turnover. Nat. Struct. Mol. Biol. 2004;11:121–127. - PubMed
-
- Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nat. Rev. Mol. Cell. Biol. 2007;8:113–126. - PubMed
-
- Coller J, Parker R. Eukaryotic mRNA decapping. Annu. Rev. Biochem. 2004;73:861–890. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

