Genome sequencing of the lizard parasite Leishmania tarentolae reveals loss of genes associated to the intracellular stage of human pathogenic species
- PMID: 21998295
- PMCID: PMC3273817
- DOI: 10.1093/nar/gkr834
Genome sequencing of the lizard parasite Leishmania tarentolae reveals loss of genes associated to the intracellular stage of human pathogenic species
Abstract
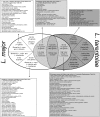
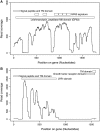
The Leishmania tarentolae Parrot-TarII strain genome sequence was resolved to an average 16-fold mean coverage by next-generation DNA sequencing technologies. This is the first non-pathogenic to humans kinetoplastid protozoan genome to be described thus providing an opportunity for comparison with the completed genomes of pathogenic Leishmania species. A high synteny was observed between all sequenced Leishmania species. A limited number of chromosomal regions diverged between L. tarentolae and L. infantum, while remaining syntenic to L. major. Globally, >90% of the L. tarentolae gene content was shared with the other Leishmania species. We identified 95 predicted coding sequences unique to L. tarentolae and 250 genes that were absent from L. tarentolae. Interestingly, many of the latter genes were expressed in the intracellular amastigote stage of pathogenic species. In addition, genes coding for products involved in antioxidant defence or participating in vesicular-mediated protein transport were underrepresented in L. tarentolae. In contrast to other Leishmania genomes, two gene families were expanded in L. tarentolae, namely the zinc metallo-peptidase surface glycoprotein GP63 and the promastigote surface antigen PSA31C. Overall, L. tarentolae's gene content appears better adapted to the promastigote insect stage rather than the amastigote mammalian stage.
Figures





References
-
- Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. - PubMed
-
- Noyes HA, Chance ML, Croan DG, Ellis JT. Leishmania (sauroleishmania): A comment on classification. Parasitol. Today. 1998;14:167. - PubMed
-
- Simpson L, Holz G. The status of Leishmania tarentolae/Trypanosoma platydactyli. Parasitol. Today. 1988;4:115–118. - PubMed
-
- Elwasila M. Leishmania tarentolae Wenyon, 1921 from the gecko Tarentola annularis in the Sudan. Parasitol. Res. 1988;74:591–592. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

