The molecular basis of folate salvage in Plasmodium falciparum: characterization of two folate transporters
- PMID: 21998306
- PMCID: PMC3247980
- DOI: 10.1074/jbc.M111.286054
The molecular basis of folate salvage in Plasmodium falciparum: characterization of two folate transporters
Abstract
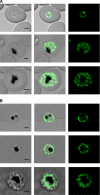
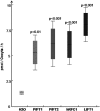
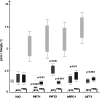
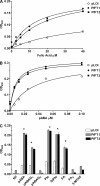
Tetrahydrofolates are essential cofactors for DNA synthesis and methionine metabolism. Malaria parasites are capable both of synthesizing tetrahydrofolates and precursors de novo and of salvaging them from the environment. The biosynthetic route has been studied in some detail over decades, whereas the molecular mechanisms that underpin the salvage pathway lag behind. Here we identify two functional folate transporters (named PfFT1 and PfFT2) and delineate unexpected substrate preferences of the folate salvage pathway in Plasmodium falciparum. Both proteins are localized in the plasma membrane and internal membranes of the parasite intra-erythrocytic stages. Transport substrates include folic acid, folinic acid, the folate precursor p-amino benzoic acid (pABA), and the human folate catabolite pABAG(n). Intriguingly, the major circulating plasma folate, 5-methyltetrahydrofolate, was a poor substrate for transport via PfFT2 and was not transported by PfFT1. Transport of all folates studied was inhibited by probenecid and methotrexate. Growth rescue in Escherichia coli and antifolate antagonism experiments in P. falciparum indicate that functional salvage of 5-methyltetrahydrofolate is detectable but trivial. In fact pABA was the only effective salvage substrate at normal physiological levels. Because pABA is neither synthesized nor required by the human host, pABA metabolism may offer opportunities for chemotherapeutic intervention.
Figures
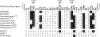

References
-
- Fox J. T., Stover P. J. (2008) in Folic Acid and Folates (Litwack G., ed.) pp 1–44, Academic Press, Inc., New York
-
- Gregson A., Plowe C. V. (2005) Pharmacol. Rev. 57, 117–145 - PubMed
-
- Tan-ariya P., Brockelman C. R. (1983) J. Parasitol. 69, 353–359 - PubMed
-
- Tan-ariya P., Brockelman C. R., Menabandhu C. (1987) Am. J. Trop. Med. Hyg. 37, 42–48 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

