Functional properties of a newly identified C-terminal splice variant of Cav1.3 L-type Ca2+ channels
- PMID: 21998310
- PMCID: PMC3234942
- DOI: 10.1074/jbc.M111.269951
Functional properties of a newly identified C-terminal splice variant of Cav1.3 L-type Ca2+ channels
Abstract
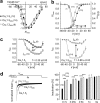
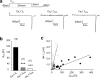
An intramolecular interaction between a distal (DCRD) and a proximal regulatory domain (PCRD) within the C terminus of long Ca(v)1.3 L-type Ca(2+) channels (Ca(v)1.3(L)) is a major determinant of their voltage- and Ca(2+)-dependent gating kinetics. Removal of these regulatory domains by alternative splicing generates Ca(v)1.3(42A) channels that activate at a more negative voltage range and exhibit more pronounced Ca(2+)-dependent inactivation. Here we describe the discovery of a novel short splice variant (Ca(v)1.3(43S)) that is expressed at high levels in the brain but not in the heart. It lacks the DCRD but, in contrast to Ca(v)1.3(42A), still contains PCRD. When expressed together with α2δ1 and β3 subunits in tsA-201 cells, Ca(v)1.3(43S) also activated at more negative voltages like Ca(v)1.3(42A) but Ca(2+)-dependent inactivation was less pronounced. Single channel recordings revealed much higher channel open probabilities for both short splice variants as compared with Ca(v)1.3(L). The presence of the proximal C terminus in Ca(v)1.3(43S) channels preserved their modulation by distal C terminus-containing Ca(v)1.3- and Ca(v)1.2-derived C-terminal peptides. Removal of the C-terminal modulation by alternative splicing also induced a faster decay of Ca(2+) influx during electrical activities mimicking trains of neuronal action potentials. Our findings extend the spectrum of functionally diverse Ca(v)1.3 L-type channels produced by tissue-specific alternative splicing. This diversity may help to fine tune Ca(2+) channel signaling and, in the case of short variants lacking a functional C-terminal modulation, prevent excessive Ca(2+) accumulation during burst firing in neurons. This may be especially important in neurons that are affected by Ca(2+)-induced neurodegenerative processes.
Figures



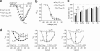
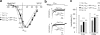

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

