CD4+ T cells and complement independently mediate graft ischemia in the rejection of mouse orthotopic tracheal transplants
- PMID: 21998328
- PMCID: PMC3243047
- DOI: 10.1161/CIRCRESAHA.111.250167
CD4+ T cells and complement independently mediate graft ischemia in the rejection of mouse orthotopic tracheal transplants
Abstract
Rationale: While microvascular injury is associated with chronic rejection, the cause of tissue ischemia during alloimmune injury is not yet elucidated.
Objective: We investigated the contribution of T lymphocytes and complement to microvascular injury-associated ischemia during acute rejection of mouse tracheal transplants.
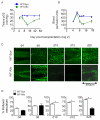
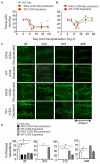
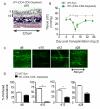
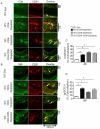
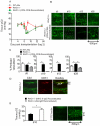
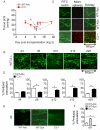
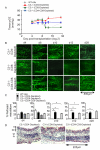
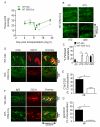
Methods and results: Using novel techniques to assess microvascular integrity and function, we evaluated how lymphocyte subsets and complement specifically affect microvascular perfusion and tissue oxygenation in MHC-mismatched transplants. To characterize T cell effects on microvessel loss and recovery, we transplanted functional airway grafts in the presence and absence of CD4(+) and CD8(+) T cells. To establish the contribution of complement-mediated injury to the allograft microcirculation, we transplanted C3-deficient and C3-inhibited recipients. We demonstrated that CD4(+) T cells and complement are independently sufficient to cause graft ischemia. CD8(+) T cells were required for airway neovascularization to occur following CD4-mediated rejection. Activation of antibody-dependent complement pathways mediated tissue ischemia even in the absence of cellular rejection. Complement inhibition by CR2-Crry attenuated graft hypoxia, complement/antibody deposition on vascular endothelium and promoted vascular perfusion by enhanced angiogenesis. Finally, there was a clear relationship between the burden of tissue hypoxia (ischemia×time duration) and the development of subsequent airway remodeling.
Conclusions: These studies demonstrated that CD4(+) T cells and complement operate independently to cause transplant ischemia during acute rejection and that sustained ischemia is a precursor to chronic rejection.
Figures
References
-
- Libby P, Pober JS. Chronic rejection. Immunity. 2001;14(4):387–397. - PubMed
-
- Ozdemir BH, Demirhan B, Ozdemir FN, Dalgic A, Haberal M. The role of microvascular injury on steroid and OKT3 response in renal allograft rejection. Transplantation. 2004;78(5):734–740. - PubMed
-
- Luckraz H, Goddard M, McNeil K, Atkinson C, Charman SC, Stewart S, Wallwork J. Microvascular changes in small airways predispose to obliterative bronchiolitis after lung transplantation. J Heart Lung Transplant. 2004;23(5):527–531. - PubMed
-
- Luckraz H, Goddard M, McNeil K, Atkinson C, Sharples LD, Wallwork J. Is obliterative bronchiolitis in lung transplantation associated with microvascular damage to small airways? Ann Thorac Surg. 2006;82(4):1212–1218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

