Fifth-generation digital immunoassay for prostate-specific antigen by single molecule array technology
- PMID: 21998342
- PMCID: PMC3402036
- DOI: 10.1373/clinchem.2011.169540
Fifth-generation digital immunoassay for prostate-specific antigen by single molecule array technology
Abstract
Background: Measurement of prostate-specific antigen (PSA) in prostate cancer patients following radical prostatectomy (RP) has been hindered by the limit of quantification of available assays. Because radical prostatectomy removes the tissue responsible for PSA production, postsurgical PSA is typically undetectable with current assay methods. Evidence suggests, however, that more sensitive determination of PSA status following RP could improve assessment of patient prognosis and response to treatment and better target secondary therapy for those who may benefit most. We developed an investigational digital immunoassay with a limit of quantification 2 logs lower than current ultrasensitive third-generation PSA assays.
Methods: We developed reagents for a bead-based ELISA for use with high-density arrays of femtoliter-volume wells. Anti-PSA capture beads with immunocomplexes and associated enzyme labels were singulated within the wells of the arrays and interrogated for the presence of enzymatic product. We characterized analytical performance, compared its accuracy with a commercially available test, and analyzed longitudinal serum samples from a pilot study of 33 RP patients.
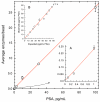
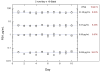
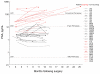
Results: The assay exhibited a functional sensitivity (20% interassay CV) <0.05 pg/mL, total imprecision <10% from 1 to 50 pg/mL, and excellent agreement with the comparator method. All RP samples were well within the assay measurement capability. PSA concentrations following surgery were found to be predictive of prostate cancer recurrence risk over 5 years.
Conclusions: The robust 2-log improvement in limit of quantification relative to current ultrasensitive assays and the validated analytical performance of the assay allow for accurate assessment of PSA status after RP.
Figures



References
-
- Oesterling JE. Prostate-specific antigen: A critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991;145:907. - PubMed
-
- Diamandis EP. Prostate specific antigen—its usefulness in clinical medicine. Trends Endocrinol Metab. 1998;9:310–316. - PubMed
-
- Gunnar A. Second-Line Therapy after Radical Prostatectomy Failure: For Whom? When? How? Eur Urol. 2007;51:1155–58. - PubMed
-
- Bock JL, Klee GG. How sensitive is a prostate-specific antigen measurement? How sensitive does it need to be? Arch Pathol Lab Med. 2004;128:341–343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

