Diabetes impairs hematopoietic stem cell mobilization by altering niche function
- PMID: 21998408
- PMCID: PMC3754876
- DOI: 10.1126/scitranslmed.3002191
Diabetes impairs hematopoietic stem cell mobilization by altering niche function
Abstract
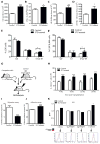
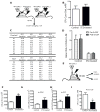
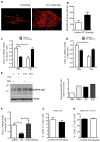
Success with transplantation of autologous hematopoietic stem and progenitor cells (HSPCs) in patients depends on adequate collection of these cells after mobilization from the bone marrow niche by the cytokine granulocyte colony-stimulating factor (G-CSF). However, some patients fail to achieve sufficient HSPC mobilization. Retrospective analysis of bone marrow transplant patient records revealed that diabetes correlated with poor mobilization of CD34+ HSPCs. In mouse models of type 1 and type 2 diabetes (streptozotocin-induced and db/db mice, respectively), we found impaired egress of murine HSPCs from the bone marrow after G-CSF treatment. Furthermore, HSPCs were aberrantly localized in the marrow niche of the diabetic mice, and abnormalities in the number and function of sympathetic nerve termini were associated with this mislocalization. Aberrant responses to β-adrenergic stimulation of the bone marrow included an inability of marrow mesenchymal stem cells expressing the marker nestin to down-modulate the chemokine CXCL12 in response to G-CSF treatment (mesenchymal stem cells are reported to be critical for HSPC mobilization). The HSPC mobilization defect was rescued by direct pharmacological inhibition of the interaction of CXCL12 with its receptor CXCR4 using the drug AMD3100. These data suggest that there are diabetes-induced changes in bone marrow physiology and microanatomy and point to a potential intervention to overcome poor HSPC mobilization in diabetic patients.
Conflict of interest statement
Figures



References
-
- Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med. 2007;357:1472–1475. - PubMed
-
- Thomas ED, Buckner CD, Sanders JE, Papayannopoulou T, Borgna-Pignatti C, De Stefano P, Sullivan KM, Clift RA, Storb R. Marrow transplantation for thalassaemia. Lancet. 1982;2:227–229. - PubMed
-
- Rick O, Siegert W, Schwella N, Dubiel M, Krusch A, Beyer J. High-dose chemotherapy as salvage treatment for seminoma. Bone Marrow Transplant. 2002;30:157–160. - PubMed
-
- Van Wijmeersch B, Sprangers B, Dubois B, Waer M, Billiau AD. Autologous and allogeneic hematopoietic stem cell transplantation for Multiple Sclerosis: perspective on mechanisms of action. J Neuroimmunol. 2008;197:89–98. - PubMed
-
- Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL044851/HL/NHLBI NIH HHS/United States
- HL097748/HL/NHLBI NIH HHS/United States
- R01 HL097819/HL/NHLBI NIH HHS/United States
- U01 HL100402/HL/NHLBI NIH HHS/United States
- HL097794/HL/NHLBI NIH HHS/United States
- R01 HL097794/HL/NHLBI NIH HHS/United States
- R01DK056638/DK/NIDDK NIH HHS/United States
- R01 DK056638/DK/NIDDK NIH HHS/United States
- DK050234/DK/NIDDK NIH HHS/United States
- R01HL097819/HL/NHLBI NIH HHS/United States
- R01 HL069438/HL/NHLBI NIH HHS/United States
- HL100402/HL/NHLBI NIH HHS/United States
- U01HL100402/HL/NHLBI NIH HHS/United States
- 5T32HL007623-24/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

