High-dose valacyclovir HSV-2 suppression results in greater reduction in plasma HIV-1 levels compared with standard dose acyclovir among HIV-1/HSV-2 coinfected persons: a randomized, crossover trial
- PMID: 21998479
- PMCID: PMC3247811
- DOI: 10.1093/infdis/jir649
High-dose valacyclovir HSV-2 suppression results in greater reduction in plasma HIV-1 levels compared with standard dose acyclovir among HIV-1/HSV-2 coinfected persons: a randomized, crossover trial
Abstract
Background: Standard-dose HSV-2 suppressive therapy (acyclovir 400 mg twice daily) reduces plasma HIV-1 levels by 0.25-0.50 log(10) copies/mL. It is not known if higher doses might further suppress HIV-1 levels.
Methods: We enrolled 32 HIV-1/HSV-2 dually infected Kenyan individuals who were not on antiretroviral therapy (ART) into a randomized, crossover trial of 2 dosing regimens of HSV-2 suppression: valacyclovir 1.5 g vs acyclovir 400 mg, both twice daily for 12 weeks, then a 2-week washout, and then the alternative for 12 weeks. Weekly plasma HIV-1 RNA quantity was measured (ClinicalTrials.gov number NCT01026454).
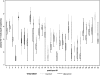
Results: Mean plasma HIV-1 levels were significantly lower on valacyclovir compared with acyclovir: 2.94 vs 3.56 log(10) copies/mL, an average difference of 0.62 log(10) copies/mL (95% confidence interval [CI]: -0.68, -0.55; P < .001), a 76% decrease. Valacyclovir resulted in a 1.23 log(10) copies/mL decrease compared with baseline HIV-1 levels without HSV-2 suppression. Adherence was similar (99.4% of dispensed study tablets taken), and high-dose valacyclovir was well tolerated.
Conclusions: High-dose valacyclovir reduced plasma HIV-1 viral levels by 0.62 log(10) copies/mL compared with standard-dose acyclovir. The potential for higher-dose HSV-2 suppressive therapy to slow HIV-1 disease progression and reduce HIV-1 infectiousness among HIV-1/HSV-2 coinfected persons not yet eligible for ART warrants further evaluation.
Figures

Comment in
-
Time to refocus on HSV interventions for HIV prevention?J Infect Dis. 2011 Dec 15;204(12):1822-6. doi: 10.1093/infdis/jir653. Epub 2011 Oct 12. J Infect Dis. 2011. PMID: 21998480 Free PMC article. No abstract available.
Similar articles
-
High-dose valacyclovir decreases plasma HIV-1 RNA more than standard-dose acyclovir in persons coinfected with HIV-1 and HSV-2: a randomized crossover trial.J Acquir Immune Defic Syndr. 2013 Jun 1;63(2):201-8. doi: 10.1097/QAI.0b013e3182928eea. J Acquir Immune Defic Syndr. 2013. PMID: 23542637 Free PMC article. Clinical Trial.
-
Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial.J Infect Dis. 2007 Nov 15;196(10):1500-8. doi: 10.1086/522523. Epub 2007 Oct 31. J Infect Dis. 2007. PMID: 18008230 Clinical Trial.
-
Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus.N Engl J Med. 2007 Feb 22;356(8):790-9. doi: 10.1056/NEJMoa062607. N Engl J Med. 2007. PMID: 17314338 Clinical Trial.
-
Valacyclovir for herpes simplex virus infection: long-term safety and sustained efficacy after 20 years' experience with acyclovir.J Infect Dis. 2002 Oct 15;186 Suppl 1:S40-6. doi: 10.1086/342966. J Infect Dis. 2002. PMID: 12353186 Review.
-
Prevention of perinatal herpes: prophylactic antiviral therapy?Clin Obstet Gynecol. 1999 Mar;42(1):134-48; quiz 174-5. doi: 10.1097/00003081-199903000-00018. Clin Obstet Gynecol. 1999. PMID: 10073307 Review.
Cited by
-
Combination therapy synergism prediction for virus treatment using machine learning models.PLoS One. 2024 Sep 4;19(9):e0309733. doi: 10.1371/journal.pone.0309733. eCollection 2024. PLoS One. 2024. PMID: 39231124 Free PMC article.
-
Infectious co-factors in HIV-1 transmission herpes simplex virus type-2 and HIV-1: new insights and interventions.Curr HIV Res. 2012 Apr;10(3):228-37. doi: 10.2174/157016212800618156. Curr HIV Res. 2012. PMID: 22384842 Free PMC article. Review.
-
Medical male circumcision and herpes simplex virus 2 acquisition: posttrial surveillance in Kisumu, Kenya.J Infect Dis. 2013 Dec 1;208(11):1869-76. doi: 10.1093/infdis/jit371. Epub 2013 Jul 30. J Infect Dis. 2013. PMID: 23901089 Free PMC article. Clinical Trial.
-
Design and implementation of a randomized crossover study of valproic acid and antiretroviral therapy to reduce the HIV reservoir.HIV Clin Trials. 2012 Nov-Dec;13(6):301-7. doi: 10.1310/hct1306-301. HIV Clin Trials. 2012. PMID: 23195668 Free PMC article. Clinical Trial.
-
The Role of Sexually Transmitted Infections in HIV-1 Progression: A Comprehensive Review of the Literature.J Sex Transm Dis. 2013;2013:176459. doi: 10.1155/2013/176459. Epub 2013 Jun 24. J Sex Transm Dis. 2013. PMID: 26316953 Free PMC article. Review.
References
-
- Gwanzura L, McFarland W, Alexander D, Burke RL, Katzenstein D. Association between human immunodeficiency virus and herpes simplex virus type 2 seropositivity among male factory workers in Zimbabwe. J Infect Dis. 1998;177:481–4. - PubMed
-
- Celum CL, Robinson NJ, Cohen MS. Potential effect of HIV type 1 antiretroviral and herpes simplex virus type 2 antiviral therapy on transmission and acquisition of HIV type 1 infection. J Infect Dis. 2005;191(Suppl 1):S107–14. - PubMed
-
- McClelland RS, Wang CC, Overbaugh J, et al. Association between cervical shedding of herpes simplex virus and HIV-1. AIDS. 2002;16:2425–30. - PubMed
-
- Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis. 2002;186:1718–25. - PubMed
-
- Conant MA, Schacker TW, Murphy RL, Gold J, Crutchfield LT, Crooks RJ. Valaciclovir versus aciclovir for herpes simplex virus infection in HIV-infected individuals: two randomized trials. Int J STD AIDS. 2002;13:12–21. - PubMed

