Coexistence of lateral and co-tuned inhibitory configurations in cortical networks
- PMID: 21998561
- PMCID: PMC3188483
- DOI: 10.1371/journal.pcbi.1002161
Coexistence of lateral and co-tuned inhibitory configurations in cortical networks
Abstract
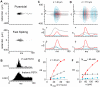
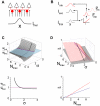
The responses of neurons in sensory cortex depend on the summation of excitatory and inhibitory synaptic inputs. How the excitatory and inhibitory inputs scale with stimulus depends on the network architecture, which ranges from the lateral inhibitory configuration where excitatory inputs are more narrowly tuned than inhibitory inputs, to the co-tuned configuration where both are tuned equally. The underlying circuitry that gives rise to lateral inhibition and co-tuning is yet unclear. Using large-scale network simulations with experimentally determined connectivity patterns and simulations with rate models, we show that the spatial extent of the input determined the configuration: there was a smooth transition from lateral inhibition with narrow input to co-tuning with broad input. The transition from lateral inhibition to co-tuning was accompanied by shifts in overall gain (reduced), output firing pattern (from tonic to phasic) and rate-level functions (from non-monotonic to monotonically increasing). The results suggest that a single cortical network architecture could account for the extended range of experimentally observed response types between the extremes of lateral inhibitory versus co-tuned configurations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Volkov IO, Galazjuk AV. Formation of spike response to sound tones in cat auditory cortex neurons: interaction of excitatory and inhibitory effects. Neuroscience. 1991;43:307–321. - PubMed
-
- Wang X, Lu T, Snider RK, Liang L. Sustained firing in auditory cortex evoked by preferred stimuli. Nature. 2005;435:341–346. - PubMed
-
- Schreiner CE, Mendelson JR, Sutter ML. Functional topography of cat primary auditory cortex: representation of tone intensity. Exp Brain Res. 1992;92:105–122. - PubMed
-
- Calford MB, Semple MN. Monaural inhibition in cat auditory cortex. J Neurophysiol. 1995;73:1876–1891. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

