Model cortical association fields account for the time course and dependence on target complexity of human contour perception
- PMID: 21998562
- PMCID: PMC3188484
- DOI: 10.1371/journal.pcbi.1002162
Model cortical association fields account for the time course and dependence on target complexity of human contour perception
Erratum in
- PLoS Comput Biol. 2011 Oct;7(10). doi: 10.1371/annotation/35890214-d064-4b76-8bfc-5ac1ab07c8b8. Kenyon, Garret T [corrected to Kenyon, Garrett T] doi: 10.1371/annotation/35890214-d064-4b76-8bfc-5ac1ab07c8b8
Abstract
Can lateral connectivity in the primary visual cortex account for the time dependence and intrinsic task difficulty of human contour detection? To answer this question, we created a synthetic image set that prevents sole reliance on either low-level visual features or high-level context for the detection of target objects. Rendered images consist of smoothly varying, globally aligned contour fragments (amoebas) distributed among groups of randomly rotated fragments (clutter). The time course and accuracy of amoeba detection by humans was measured using a two-alternative forced choice protocol with self-reported confidence and variable image presentation time (20-200 ms), followed by an image mask optimized so as to interrupt visual processing. Measured psychometric functions were well fit by sigmoidal functions with exponential time constants of 30-91 ms, depending on amoeba complexity. Key aspects of the psychophysical experiments were accounted for by a computational network model, in which simulated responses across retinotopic arrays of orientation-selective elements were modulated by cortical association fields, represented as multiplicative kernels computed from the differences in pairwise edge statistics between target and distractor images. Comparing the experimental and the computational results suggests that each iteration of the lateral interactions takes at least [Formula: see text] ms of cortical processing time. Our results provide evidence that cortical association fields between orientation selective elements in early visual areas can account for important temporal and task-dependent aspects of the psychometric curves characterizing human contour perception, with the remaining discrepancies postulated to arise from the influence of higher cortical areas.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

 . Top row: Targets; amoeba complexity increases with increasing numbers of radial frequencies. Clutter was constructed by randomly rotating groups of amoeba contour fragments. Bottom row: Distractors; only clutter fragments are present.
. Top row: Targets; amoeba complexity increases with increasing numbers of radial frequencies. Clutter was constructed by randomly rotating groups of amoeba contour fragments. Bottom row: Distractors; only clutter fragments are present.
 ) from vertical to mitigate aliasing effects. The color of each edge was set proportional to its co-occurrence-based support. The color scale ranges from blue (negative values) to white (zero) to red (positive values). Left panel: Co-occurrence statistics compiled from
) from vertical to mitigate aliasing effects. The color of each edge was set proportional to its co-occurrence-based support. The color scale ranges from blue (negative values) to white (zero) to red (positive values). Left panel: Co-occurrence statistics compiled from  target images. Center panel: Co-occurrence statistics compiled from
target images. Center panel: Co-occurrence statistics compiled from  distractor images. Right panel: ODD kernel, given by the difference in co-occurrence statistics between target and distractor kernels. Bottom Row: Subfields extracted from the middle of the upper left quadrant (as indicated by black boxes in the top row figures), shown on an expanded scale to better visualize the difference in co-occurrence statistics between target and distractor images. Alignment of edges in target images is mostly cocircular whereas alignment is mostly random in distractor images, accounting for the fine structure in the corresponding section of the ODD kernel.
distractor images. Right panel: ODD kernel, given by the difference in co-occurrence statistics between target and distractor kernels. Bottom Row: Subfields extracted from the middle of the upper left quadrant (as indicated by black boxes in the top row figures), shown on an expanded scale to better visualize the difference in co-occurrence statistics between target and distractor images. Alignment of edges in target images is mostly cocircular whereas alignment is mostly random in distractor images, accounting for the fine structure in the corresponding section of the ODD kernel.
 ). Right column: Gray-scale natural image (the standard computer vision test image “Lena”) after applying a hard Difference of Gaussians (DoG) filter to enhance edges. Top row: Raw retinal input. Second row: Responses of orientation-selective elements before any lateral interactions (
). Right column: Gray-scale natural image (the standard computer vision test image “Lena”) after applying a hard Difference of Gaussians (DoG) filter to enhance edges. Top row: Raw retinal input. Second row: Responses of orientation-selective elements before any lateral interactions ( ). To aid visualization, the activity of the maximally responding orientation-selective element at each pixel location is depicted as a gray-scale intensity. Rows 3-6: Activity after
). To aid visualization, the activity of the maximally responding orientation-selective element at each pixel location is depicted as a gray-scale intensity. Rows 3-6: Activity after  iterations of the multiplicative ODD kernel, as labeled. For each iteration, activity was multiplied by the local support, computed via linear convolution of the previous output activity with the ODD kernel. Lateral interactions tended to support smooth contours, particularly those arising from amoeba segments, while suppressing clutter or background detail.
iterations of the multiplicative ODD kernel, as labeled. For each iteration, activity was multiplied by the local support, computed via linear convolution of the previous output activity with the ODD kernel. Lateral interactions tended to support smooth contours, particularly those arising from amoeba segments, while suppressing clutter or background detail.
 test target images. Blue bins: Total activity histograms for all
test target images. Blue bins: Total activity histograms for all  test distractor images. The degree that the two distributions overlap is shown as the gray shaded area, which provides a measure of whether total luminance can be used to distinguish targets from distractors. The percentage in each shaded area shows the approximate lower bound amount of overlap of the two histograms, for comparison. Top row: Total summed activity over all retinal pixels. Little, if any bias between target and distractor images was evident in the input black and white images as there is nearly complete overlap between the distributions. Subsequent rows: Total activity histograms summed over all orientation-selective elements. Second row: Bottom-up responses prior to any lateral interactions. Third - sixth rows: Total activity histograms after
test distractor images. The degree that the two distributions overlap is shown as the gray shaded area, which provides a measure of whether total luminance can be used to distinguish targets from distractors. The percentage in each shaded area shows the approximate lower bound amount of overlap of the two histograms, for comparison. Top row: Total summed activity over all retinal pixels. Little, if any bias between target and distractor images was evident in the input black and white images as there is nearly complete overlap between the distributions. Subsequent rows: Total activity histograms summed over all orientation-selective elements. Second row: Bottom-up responses prior to any lateral interactions. Third - sixth rows: Total activity histograms after  -
-  iterations of the multiplicative ODD kernel, respectively. Total summed activity became progressively more separable with additional iterations, as evinced by a decrease in the overlapping areas.
iterations of the multiplicative ODD kernel, respectively. Total summed activity became progressively more separable with additional iterations, as evinced by a decrease in the overlapping areas.
 ms and
ms and  ms, followed by an optimized
ms, followed by an optimized  ms mask generated from randomly rotated groups of target and distractor segments. Subjects indicated which side contained the target object (amoeba) using a computer mouse to click along a horizontal slider bar. Clicking far to the left or right indicated strong confidence that the corresponding side contained the target; clicking close to the center indicated weak confidence. A narrow gap in the center forced subjects to choose between left and right.
ms mask generated from randomly rotated groups of target and distractor segments. Subjects indicated which side contained the target object (amoeba) using a computer mouse to click along a horizontal slider bar. Clicking far to the left or right indicated strong confidence that the corresponding side contained the target; clicking close to the center indicated weak confidence. A narrow gap in the center forced subjects to choose between left and right.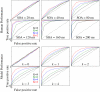
 , respectively. Bottom two rows: ROC curves for model cortical association fields computed from total activity histograms.
, respectively. Bottom two rows: ROC curves for model cortical association fields computed from total activity histograms.
 radial frequencies.
radial frequencies.
References
-
- Velisavljević L, Elder JH. Cue dynamics underlying rapid detection of animals in natural scenes. J Vision. 2009;9 - PubMed
-
- Field DJ, Hayes A, Hess RF. Contour integration by the human visual system: Evidence for a local “association field”. Vision Res. 1993;33:173–193. - PubMed
-
- Loffler G. Perception of contours and shapes: Low and intermediate stage mechanisms. Vision Res. 2008;48:2106–2127. - PubMed
-
- Hess R, Field D. Integration of contours: new insights. Trends Cogn Sci. 1999;3:480–486. - PubMed
-
- Fitzpatrick D. Seeing beyond the receptive field in primary visual cortex. Curr Opin in Neurobiol. 2000;10:438–443. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

