Fumarate reductase activity maintains an energized membrane in anaerobic Mycobacterium tuberculosis
- PMID: 21998585
- PMCID: PMC3188519
- DOI: 10.1371/journal.ppat.1002287
Fumarate reductase activity maintains an energized membrane in anaerobic Mycobacterium tuberculosis
Abstract
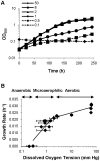
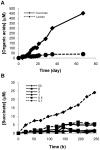
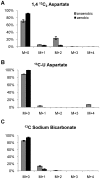
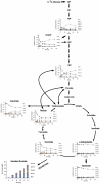
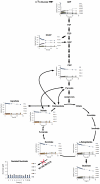
Oxygen depletion of Mycobacterium tuberculosis engages the DosR regulon that coordinates an overall down-regulation of metabolism while up-regulating specific genes involved in respiration and central metabolism. We have developed a chemostat model of M. tuberculosis where growth rate was a function of dissolved oxygen concentration to analyze metabolic adaptation to hypoxia. A drop in dissolved oxygen concentration from 50 mmHg to 0.42 mmHg led to a 2.3 fold decrease in intracellular ATP levels with an almost 70-fold increase in the ratio of NADH/NAD(+). This suggests that re-oxidation of this co-factor becomes limiting in the absence of a terminal electron acceptor. Upon oxygen limitation genes involved in the reverse TCA cycle were upregulated and this upregulation was associated with a significant accumulation of succinate in the extracellular milieu. We confirmed that this succinate was produced by a reversal of the TCA cycle towards the non-oxidative direction with net CO(2) incorporation by analysis of the isotopomers of secreted succinate after feeding stable isotope ((13)C) labeled precursors. This showed that the resulting succinate retained both carbons lost during oxidative operation of the TCA cycle. Metabolomic analyses of all glycolytic and TCA cycle intermediates from (13)C-glucose fed cells under aerobic and anaerobic conditions showed a clear reversal of isotope labeling patterns accompanying the switch from normoxic to anoxic conditions. M. tuberculosis encodes three potential succinate-producing enzymes including a canonical fumarate reductase which was highly upregulated under hypoxia. Knockout of frd, however, failed to reduce succinate accumulation and gene expression studies revealed a compensatory upregulation of two homologous enzymes. These major realignments of central metabolism are consistent with a model of oxygen-induced stasis in which an energized membrane is maintained by coupling the reductive branch of the TCA cycle to succinate secretion. This fermentative process may offer unique targets for the treatment of latent tuberculosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. - PubMed
-
- Boshoff HI, Barry CE., Iii Is the mycobacterial cell wall a hopeless drug target for latent tuberculosis? Drug Discovery Today: Disease Mechanisms. 2006;3:237–245.
-
- Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49:1–51. - PubMed
-
- Lee SW, Jang YS, Park CM, Kang HY, Koh WJ, et al. The role of chest CT scanning in TB outbreak investigation. Chest. 2010;137:1057–1064. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

