The anti-interferon activity of conserved viral dUTPase ORF54 is essential for an effective MHV-68 infection
- PMID: 21998588
- PMCID: PMC3188543
- DOI: 10.1371/journal.ppat.1002292
The anti-interferon activity of conserved viral dUTPase ORF54 is essential for an effective MHV-68 infection
Abstract
Gammaherpesviruses such as KSHV and EBV establish lifelong persistent infections through latency in lymphocytes. These viruses have evolved several strategies to counteract the various components of the innate and adaptive immune systems. We conducted an unbiased screen using the genetically and biologically related virus, MHV-68, to find viral ORFs involved in the inhibition of type I interferon signaling and identified a conserved viral dUTPase, ORF54. Here we define the contribution of ORF54 in type I interferon inhibition by ectopic expression and through the use of genetically modified MHV-68. ORF54 and an ORF54 lacking dUTPase enzymatic activity efficiently inhibit type I interferon signaling by inducing the degradation of the type I interferon receptor protein IFNAR1. Subsequently, we show in vitro that the lack of ORF54 causes a reduction in lytic replication in the presence of type I interferon signaling. Investigation of the physiological consequence of IFNAR1 degradation and importance of ORF54 during MHV-68 in vivo infection demonstrates that ORF54 has an even greater impact on persistent infection than on lytic replication. MHV-68 lacking ORF54 expression is unable to efficiently establish latent infection in lymphocytes, although it replicates relatively normally in lung tissues. However, infection of IFNAR-/- mice alleviates this phenotype, emphasizing the specific role of ORF54 in type I interferon inhibition. Infection of mice and cells by a recombinant MHV-68 virus harboring a site specific mutation in ORF54 rendering the dUTPase inactive demonstrates that dUTPase enzymatic activity is not required for anti-interferon function of ORF54. Moreover, we find that dUTPase activity is dispensable at all stages of MHV-68 infection analyzed. Overall, our data suggest that ORF54 has evolved anti-interferon activity in addition to its dUTPase enzymatic activity, and that it is actually the anti-interferon role that renders ORF54 critical for establishing an effective persistent infection of MHV-68.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
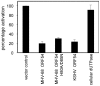



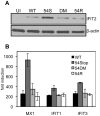
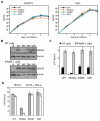

References
-
- Katze MG, He Y, Gale M., Jr Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002;2:675–687. - PubMed
-
- Mossman KL, Ashkar AA. Herpesviruses and the innate immune response. Viral Immunol. 2005;18:267–281. - PubMed
-
- Alam R, Gorska M. 3. Lymphocytes. J Allergy Clin Immunol. 2003;111:S476–485. - PubMed
-
- Chaplin DD. 1. Overview of the immune response. J Allergy Clin Immunol. 2003;111:S442–459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

