Toll-like receptor 7 controls the anti-retroviral germinal center response
- PMID: 21998589
- PMCID: PMC3188541
- DOI: 10.1371/journal.ppat.1002293
Toll-like receptor 7 controls the anti-retroviral germinal center response
Abstract
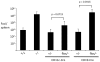
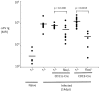
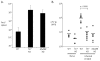
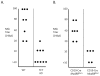
The development of vaccines that can enhance immunity to viral pathogens is an important goal. However, the innate molecular pathways that regulate the strength and quality of the immune response remain largely uncharacterized. To define the role of Toll-like receptor (TLR) signaling in control of a model retroviral pathogen, Friend virus (FV), I generated mice in which the TLR signaling adapter Myd88 was selectively deleted in dendritic cell (DC) or in B cell lineages. Deletion of Myd88 in DCs had little effect on immune control of FV, while B cell specific deletion of Myd88 caused a dramatic increase in viral infectious centers and a significantly reduced antibody response, indicating that B cell-intrinsic TLR signaling plays a crucial role, while TLR signaling in DCs is less important. I then identified the single-stranded RNA sensing protein TLR7 as being required for antibody-mediated control of FV by analyzing mice deficient in TLR7. Remarkably, B cells in infected TLR7-deficient mice upregulated CD69 and CD86 early in infection, but failed to develop into germinal center B cells. CD4 T cell responses were also attenuated in the absence of TLR7, but CD8 responses were TLR7 independent, suggesting the existence of additional pathways for detection of retroviral particles. Together these results demonstrate that the vertebrate immune system detects retroviruses in vivo via TLR7 and that this pathway regulates a key checkpoint controlling development of germinal center B cells.
Conflict of interest statement
The author has declared that no competing interests exist.
Figures




References
-
- Broder S, Gallo RC. A pathogenic retrovirus (HTLV-III) linked to AIDS. N Engl J Med. 1984;311:1292–1297. - PubMed
-
- Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983;220:868–871. - PubMed
-
- Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678–682. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

