A barcode screen for epigenetic regulators reveals a role for the NuB4/HAT-B histone acetyltransferase complex in histone turnover
- PMID: 21998594
- PMCID: PMC3188528
- DOI: 10.1371/journal.pgen.1002284
A barcode screen for epigenetic regulators reveals a role for the NuB4/HAT-B histone acetyltransferase complex in histone turnover
Abstract
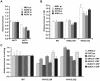
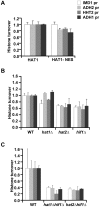
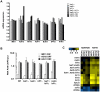
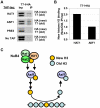
Dynamic modification of histone proteins plays a key role in regulating gene expression. However, histones themselves can also be dynamic, which potentially affects the stability of histone modifications. To determine the molecular mechanisms of histone turnover, we developed a parallel screening method for epigenetic regulators by analyzing chromatin states on DNA barcodes. Histone turnover was quantified by employing a genetic pulse-chase technique called RITE, which was combined with chromatin immunoprecipitation and high-throughput sequencing. In this screen, the NuB4/HAT-B complex, containing the conserved type B histone acetyltransferase Hat1, was found to promote histone turnover. Unexpectedly, the three members of this complex could be functionally separated from each other as well as from the known interacting factor and histone chaperone Asf1. Thus, systematic and direct interrogation of chromatin structure on DNA barcodes can lead to the discovery of genes and pathways involved in chromatin modification and dynamics.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Rando OJ, Ahmad K. Rules and regulation in the primary structure of chromatin. Curr Opin Cell Biol. 2007;19:250–256. - PubMed
-
- Henikoff S. Nucleosome destabilization in the epigenetic regulation of gene expression. Nat Rev Genet. 2008;9:15–26. - PubMed
-
- Dion MF, Kaplan T, Kim M, Buratowski S, Friedman N, et al. Dynamics of replication-independent histone turnover in budding yeast. Science. 2007;315:1405–1408. - PubMed
-
- Jamai A, Imoberdorf RM, Strubin M. Continuous histone H2B and transcription-dependent histone H3 exchange in yeast cells outside of replication. Mol Cell. 2007;25:345–355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

