Dicer1 depletion in male germ cells leads to infertility due to cumulative meiotic and spermiogenic defects
- PMID: 21998645
- PMCID: PMC3187767
- DOI: 10.1371/journal.pone.0025241
Dicer1 depletion in male germ cells leads to infertility due to cumulative meiotic and spermiogenic defects
Abstract
Background: Spermatogenesis is a complex biological process that requires a highly specialized control of gene expression. In the past decade, small non-coding RNAs have emerged as critical regulators of gene expression both at the transcriptional and post-transcriptional level. DICER1, an RNAse III endonuclease, is essential for the biogenesis of several classes of small RNAs, including microRNAs (miRNAs) and endogenous small interfering RNAs (endo-siRNAs), but is also critical for the degradation of toxic transposable elements. In this study, we investigated to which extent DICER1 is required for germ cell development and the progress of spermatogenesis in mice.
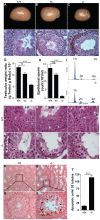
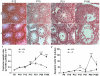
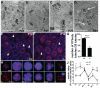
Principal findings: We show that the selective ablation of Dicer1 at the early onset of male germ cell development leads to infertility, due to multiple cumulative defects at the meiotic and post-meiotic stages culminating with the absence of functional spermatozoa. Alterations were observed in the first spermatogenic wave and include delayed progression of spermatocytes to prophase I and increased apoptosis, resulting in a reduced number of round spermatids. The transition from round to mature spermatozoa was also severely affected, since the few spermatozoa formed in mutant animals were immobile and misshapen, exhibiting morphological defects of the head and flagellum. We also found evidence that the expression of transposable elements of the SINE family is up-regulated in Dicer1-depleted spermatocytes.
Conclusions/significance: Our findings indicate that DICER1 is dispensable for spermatogonial stem cell renewal and mitotic proliferation, but is required for germ cell differentiation through the meiotic and haploid phases of spermatogenesis.
Conflict of interest statement
Figures
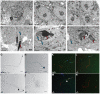
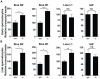
References
-
- Eddy EM. Male germ cell gene expression. Recent Prog Horm Res. 2002;57:103–128. - PubMed
-
- Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. - PubMed
-
- He Z, Kokkinaki M, Pant D, Gallicano GI, Dym M. Small RNA molecules in the regulation of spermatogenesis. Reproduction. 2009;137:901–911. - PubMed
-
- Papaioannou MD, Nef S. microRNAs in the testis: building up male fertility. J Androl. 2010;31:26–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

