Replication of Epstein-Barr virus primary infection in human tonsil tissue explants
- PMID: 21998663
- PMCID: PMC3187765
- DOI: 10.1371/journal.pone.0025490
Replication of Epstein-Barr virus primary infection in human tonsil tissue explants
Abstract
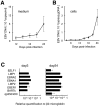
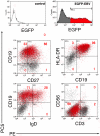
Epstein-Barr virus (EBV) may cause a variety of virus-associated diseases, but no antiviral agents have yet been developed against this virus. Animal models are thus indispensable for the pathological analysis of EBV-related infections and the elucidation of therapeutic methods. To establish a model system for the study of EBV infection, we tested the ability of B95-8 virus and recombinant EBV expressing enhanced green fluorescent protein (EGFP) to replicate in human lymphoid tissue. Human tonsil tissues that had been surgically removed during routine tonsillectomy were sectioned into small blocks and placed on top of collagen sponge gels in culture medium at the air-interface, then a cell-free viral suspension was directly applied to the top of each tissue block. Increasing levels of EBV DNA in culture medium were observed after 12-15 days through 24 days post-infection in tissue models infected with B95-8 and EGFP-EBV. Expression levels of eight EBV-associated genes in cells collected from culture medium were increased during culture. EBV-encoded small RNA-positive cells were detected in the interfollicular areas in paraffin-embedded sections. Flow cytometric analyses revealed that most EGFP(+) cells were CD3(-) CD56(-) CD19(+) HLA-DR(+), and represented both naïve (immunoglobulin D(+)) and memory (CD27(+)) B cells. Moreover, EBV replication in this model was suppressed by acyclovir treatment in a dose-dependent manner. These data suggest that this model has potential for use in the pathological analysis of local tissues at the time of primary infection, as well as for screening novel antiviral agents.
Conflict of interest statement
Figures




References
-
- Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000;343:481–492. - PubMed
-
- Alan B. Rickinson EK. Epstein-Barr virus. In: David M.Knipe PMH, editor. Fields Virology Fifth ed. Philadelphia, , PA: Lippincott Williams & Wilkins; 2007. pp. 2655–2700.
-
- Johannessen I, Crawford DH. In vivo models for Epstein-Barr virus (EBV)-associated B cell lymphoproliferative disease (BLPD). Rev Med Virol. 1999;9:263–277. - PubMed
-
- Woodford NL, Call DR, Remick DG, Rochford R. Model of angiogenesis in mice with severe combined immunodeficiency (SCID) and xenoengrafted with Epstein-Barr virus-transformed B cells. Comp Med. 2004;54:209–215. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

