C/EBPβ-Thr217 phosphorylation signaling contributes to the development of lung injury and fibrosis in mice
- PMID: 21998664
- PMCID: PMC3187778
- DOI: 10.1371/journal.pone.0025497
C/EBPβ-Thr217 phosphorylation signaling contributes to the development of lung injury and fibrosis in mice
Abstract
Background: Although C/EBPβ(ko) mice are refractory to Bleomycin-induced lung fibrosis the molecular mechanisms remain unknown. Here we show that blocking the ribosomal S-6 kinase (RSK) phosphorylation of the CCAAT/Enhancer Binding Protein (C/EBP)-β on Thr217 (a RSK phosphoacceptor) with either a single point mutation (Ala217), dominant negative transgene or a blocking peptide containing the mutated phosphoacceptor ameliorates the progression of lung injury and fibrosis induced by Bleomycin in mice.
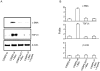
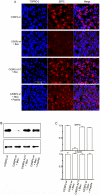
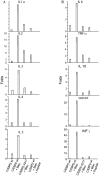
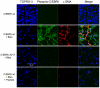
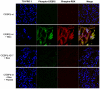
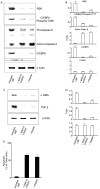
Methodology/principal findings: Mice expressing the non-phosphorylatable C/EBPβ-Ala217 transgene had a marked reduction in lung injury on day-13 after Bleomycin exposure, compared to C/EBPβ(wt) mice, judging by the decrease of CD68(+) activated monocytes/macrophages, bone marrow-derived CD45(+) cells and lung cytokines as well as by the normal surfactant protein-C expression by lung pneumocytes. On day-21 after Bleomycin treatment, C/EBPβ(wt) mice but not mice expressing the dominant negative C/EBPβ-Ala217 transgene developed severe lung fibrosis as determined by quantitative collagen assays. All mice were of identical genetic background and back-crossed to the parental wild-type inbreed FVB mice for at least ten generations. Treatment of C/EBPβ(wt) mice with a cell permeant, C/EBPβ peptide that inhibits phosphorylation of C/EBPβ on Thr217 (40 µg instilled intracheally on day-2 and day-6 after the single Bleomycin dose) also blocked the progression of lung injury and fibrosis induced by Bleomycin. Phosphorylation of human C/EBPβ on Thr266 (human homologue phosphoacceptor) was induced in collagen-activated human lung fibroblasts in culture as well as in activated lung fibroblasts in situ in lungs of patients with severe lung fibrosis but not in control lungs, suggesting that this signaling pathway may be also relevant in human lung injury and fibrosis.
Conclusions/significance: These data suggest that the RSK-C/EBPβ phosphorylation pathway may contribute to the development of lung injury and fibrosis.
Conflict of interest statement
Figures



References
-
- Crystal RG, Bitterman PB, Mossman B, Schwarz MI, Sheppard D, et al. Future Research Directions in Idiopathic Pulmonary Fibrosis: Summary of a National Heart, Lung, and Blood Institute Working Group. Am J Respir Crit Care Med. 2002;166:236–246. - PubMed
-
- Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, et al. International guidelines for the selection of lung transplant candidates: 2006 update–a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–755. - PubMed
-
- Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Res Critoical Care Med. 2006;174:810–816. - PubMed
-
- Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, et al. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176:277–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

