Praziquantel facilitates IFN-γ-producing CD8+ T cells (Tc1) and IL-17-producing CD8+ T cells (Tc17) responses to DNA vaccination in mice
- PMID: 21998665
- PMCID: PMC3187796
- DOI: 10.1371/journal.pone.0025525
Praziquantel facilitates IFN-γ-producing CD8+ T cells (Tc1) and IL-17-producing CD8+ T cells (Tc17) responses to DNA vaccination in mice
Abstract
Background: CD8(+) cytotoxic T lymphocytes (CTLs) are crucial for eliminating hepatitis B virus (HBV) infected cells. DNA vaccination, a novel therapeutic strategy for chronic virus infection, has been shown to induce CTL responses. However, accumulated data have shown that CTLs could not be effectively induced by HBV DNA vaccination.
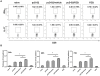
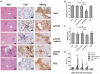
Methodology/principal findings: Here, we report that praziquantel (PZQ), an anti-schistoma drug, could act as an adjuvant to overcome the lack of potent CTL responses by HBV DNA vaccination in mice. PZQ in combination with HBV DNA vaccination augmented the induction of CD8(+) T cell-dependent and HBV-specific delayed hypersensitivity responses (DTH) in C57BL/6 mice. Furthermore, the induced CD8(+) T cells consisted of both Tc1 and Tc17 subtypes. By using IFN-γ knockout (KO) mice and IL-17 KO mice, both cytokines were found to be involved in the DTH. The relevance of these findings to HBV immunization was established in HBsAg transgenic mice, in which PZQ also augmented the induction of HBV-specific Tc1 and Tc17 cells and resulted in reduction of HBsAg positive hepatocytes. Adoptive transfer experiments further showed that PZQ-primed CD8(+) T cells from wild type mice, but not the counterpart from IFN-γ KO or IL-17 KO mice, resulted in elimination of HBsAg positive hepatocytes.
Conclusions/significance: Our results suggest that PZQ is an effective adjuvant to facilitate Tc1 and Tc17 responses to HBV DNA vaccination, inducing broad CD8(+) T cell-based immunotherapy that breaks tolerance to HBsAg.
Conflict of interest statement
Figures




Similar articles
-
Overcoming HBV immune tolerance to eliminate HBsAg-positive hepatocytes via pre-administration of GM-CSF as a novel adjuvant for a hepatitis B vaccine in HBV transgenic mice.Cell Mol Immunol. 2016 Nov;13(6):850-861. doi: 10.1038/cmi.2015.64. Epub 2015 Jul 13. Cell Mol Immunol. 2016. PMID: 26166767 Free PMC article.
-
Cimetidine synergizes with Praziquantel to enhance the immune response of HBV DNA vaccine via activating cytotoxic CD8(+) T cell.Hum Vaccin Immunother. 2014;10(6):1688-99. doi: 10.4161/hv.28517. Epub 2014 Mar 18. Hum Vaccin Immunother. 2014. PMID: 24643207 Free PMC article.
-
IL-12-based vaccination therapy reverses liver-induced systemic tolerance in a mouse model of hepatitis B virus carrier.J Immunol. 2013 Oct 15;191(8):4184-93. doi: 10.4049/jimmunol.1203449. Epub 2013 Sep 18. J Immunol. 2013. PMID: 24048897
-
DNA-mediated immunization to the hepatitis B surface antigen. Activation and entrainment of the immune response.Ann N Y Acad Sci. 1995 Nov 27;772:64-76. doi: 10.1111/j.1749-6632.1995.tb44732.x. Ann N Y Acad Sci. 1995. PMID: 8546414 Review.
-
Immunoregulation of hepatitis B virus infection--rationale and clinical application.Nagoya J Med Sci. 2012 Aug;74(3-4):217-32. Nagoya J Med Sci. 2012. PMID: 23092095 Free PMC article. Review.
Cited by
-
TIM-3 regulates innate immune cells to induce fetomaternal tolerance.J Immunol. 2013 Jan 1;190(1):88-96. doi: 10.4049/jimmunol.1202176. Epub 2012 Nov 23. J Immunol. 2013. PMID: 23180822 Free PMC article.
-
Overcoming HBV immune tolerance to eliminate HBsAg-positive hepatocytes via pre-administration of GM-CSF as a novel adjuvant for a hepatitis B vaccine in HBV transgenic mice.Cell Mol Immunol. 2016 Nov;13(6):850-861. doi: 10.1038/cmi.2015.64. Epub 2015 Jul 13. Cell Mol Immunol. 2016. PMID: 26166767 Free PMC article.
-
Cimetidine synergizes with Praziquantel to enhance the immune response of HBV DNA vaccine via activating cytotoxic CD8(+) T cell.Hum Vaccin Immunother. 2014;10(6):1688-99. doi: 10.4161/hv.28517. Epub 2014 Mar 18. Hum Vaccin Immunother. 2014. PMID: 24643207 Free PMC article.
-
CD8+ Treg cells suppress CD8+ T cell-responses by IL-10-dependent mechanism during H5N1 influenza virus infection.Eur J Immunol. 2014 Jan;44(1):103-14. doi: 10.1002/eji.201343583. Epub 2013 Nov 4. Eur J Immunol. 2014. PMID: 24114149 Free PMC article.
-
Use of praziquantel as an adjuvant enhances protection and Tc-17 responses to killed H5N1 virus vaccine in mice.PLoS One. 2012;7(4):e34865. doi: 10.1371/journal.pone.0034865. Epub 2012 Apr 18. PLoS One. 2012. PMID: 22529945 Free PMC article.
References
-
- Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. - PubMed
-
- Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. - PubMed
-
- Ganem D, Prince AM. Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. - PubMed
-
- Ferrari C, Penna A, Bertoletti A, Valli A, Antoni AD, et al. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990;145:3442–3449. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

