Functional reconstitution of a voltage-gated potassium channel in giant unilamellar vesicles
- PMID: 21998666
- PMCID: PMC3188570
- DOI: 10.1371/journal.pone.0025529
Functional reconstitution of a voltage-gated potassium channel in giant unilamellar vesicles
Abstract
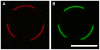
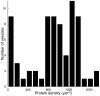
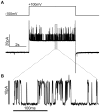
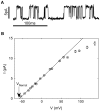
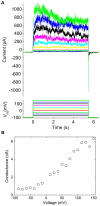
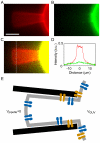
Voltage-gated ion channels are key players in cellular excitability. Recent studies suggest that their behavior can depend strongly on the membrane lipid composition and physical state. In vivo studies of membrane/channel and channel/channel interactions are challenging as membrane properties are actively regulated in living cells, and are difficult to control in experimental settings. We developed a method to reconstitute functional voltage-gated ion channels into cell-sized Giant Unilamellar Vesicles (GUVs) in which membrane composition, tension and geometry can be controlled. First, a voltage-gated potassium channel, KvAP, was purified, fluorescently labeled and reconstituted into small proteoliposomes. Small proteoliposomes were then converted into GUVs via electroformation. GUVs could be formed using different lipid compositions and buffers containing low (5 mM) or near-physiological (100 mM) salt concentrations. Protein incorporation into GUVs was characterized with quantitative confocal microscopy, and the protein density of GUVs was comparable to the small proteoliposomes from which they were formed. Furthermore, patch-clamp measurements confirmed that the reconstituted channels retained potassium selectivity and voltage-gated activation. GUVs containing functional voltage-gated ion channels will allow the study of channel activity, distribution and diffusion while controlling membrane state, and should prove a powerful tool for understanding how the membrane modulates cellular excitability.
Conflict of interest statement
Figures


References
-
- Hille B. Ion Channels of Excitable Membranes (3rd Edition) Sunderland: Sinauer Associates; 2001.
-
- Marban E. Cardiac channelopathies. Nature. 2002;415:213–8. - PubMed
-
- Ryan DP, Placek LJ. Episodic neurological channelopathies. Neuron. 2010;68:282–292. - PubMed
-
- Kullmann DM. Neurological channelopathies. Annu Rev Neurosci. 2010;33:151–172. - PubMed
-
- Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7:548–562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

