Circumsporozoite-specific T cell responses in children vaccinated with RTS,S/AS01E and protection against P falciparum clinical malaria
- PMID: 21998698
- PMCID: PMC3188575
- DOI: 10.1371/journal.pone.0025786
Circumsporozoite-specific T cell responses in children vaccinated with RTS,S/AS01E and protection against P falciparum clinical malaria
Abstract
Background: RTS,S/AS01(E) is the lead candidate pre-erythrocytic malaria vaccine. In Phase IIb field trials the safety profile was acceptable and the efficacy was 53% (95%CI 31%-72%) for protecting children against clinical malaria caused by P. falciparum. We studied CS-specific T cell responses in order to identify correlates of protection.
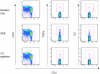
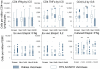
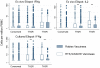
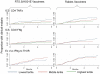
Methods and findings: We used intracellular cytokine staining (for IL2, IFNγ, and TNFα), ex-vivo ELISPOTs (IFNγ and IL2) and IFNγ cultured ELISPOT assays to characterize the CS-specific cellular responses in 407 children (5-17 months of age) in a phase IIb randomized controlled trial of RTS,S/AS01(E) (NCT00380393). RTS,S/ AS01(E) vaccinees had higher frequencies of CS-specific CD4+ T cells producing IFNγ, TNFα or IL2 compared to control vaccinees. In a multivariable analysis TNFα(+) CD4(+) T cells were independently associated with a reduced risk for clinical malaria among RTS,S/AS01(E) vaccinees (HR = 0.64, 95%CI 0.49-0.86, p = 0.002). There was a non-significant tendency towards reduced risk among control vaccinees (HR = 0.80, 95%CI 0.62-1.03, p = 0.084), albeit with lower CS-specific T cell frequencies and higher rates of clinical malaria. When data from both RTS,S/AS01(E) vaccinees and control vaccinees were combined (with adjusting for vaccination group), the HR was 0.74 (95%CI 0.62-0.89, p = 0.001). After a Bonferroni correction for multiple comparisons (n-18), the finding was still significant at p = 0.018. There was no significant correlation between cultured or ex vivo ELISPOT data and protection from clinical malaria. The combination of TNFα(+) CD4(+) T cells and anti-CS antibody statistically accounted for the protective effect of vaccination in a Cox regression model.
Conclusions: RTS,S/AS01(E) induces CS-specific Th1 T cell responses in young children living in a malaria endemic area. The combination of anti-CS antibody concentrations titers and CS-specific TNFα(+) CD4(+) T cells could account for the level of protection conferred by RTS,S/AS01(E). The correlation between CS-specific TNFα(+) CD4(+) T cells and protection needs confirmation in other datasets.
Conflict of interest statement
Figures
References
-
- Ballou WR. The development of the RTS,S malaria vaccine candidate: challenges and lessons. Parasite Immunol. 2009;31:492–500. - PubMed
-
- Bojang KA, Olodude F, Pinder M, Ofori-Anyinam O, Vigneron L, et al. Safety and immunogenicty of RTS,S/AS02A candidate malaria vaccine in Gambian children. Vaccine. 2005;23:4148–4157. - PubMed
-
- Bojang KA, Milligan PJ, Pinder M, Vigneron L, Alloueche A, et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet. 2001;358:1927–1934. - PubMed
-
- Doherty JF, Pinder M, Tornieporth N, Carton C, Vigneron L, et al. A phase I safety and immunogenicity trial with the candidate malaria vaccine RTS,S/SBAS2 in semi-immune adults in The Gambia. Am J Trop Med Hyg. 1999;61:865–868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

