3D Mapping of the SPRY2 domain of ryanodine receptor 1 by single-particle cryo-EM
- PMID: 21998699
- PMCID: PMC3187800
- DOI: 10.1371/journal.pone.0025813
3D Mapping of the SPRY2 domain of ryanodine receptor 1 by single-particle cryo-EM
Abstract
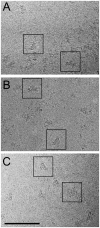
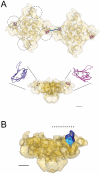
The type 1 skeletal muscle ryanodine receptor (RyR1) is principally responsible for Ca(2+) release from the sarcoplasmic reticulum and for the subsequent muscle contraction. The RyR1 contains three SPRY domains. SPRY domains are generally known to mediate protein-protein interactions, however the location of the three SPRY domains in the 3D structure of the RyR1 is not known. Combining immunolabeling and single-particle cryo-electron microscopy we have mapped the SPRY2 domain (S1085-V1208) in the 3D structure of RyR1 using three different antibodies against the SPRY2 domain. Two obstacles for the image processing procedure; limited amount of data and signal dilution introduced by the multiple orientations of the antibody bound in the tetrameric RyR1, were overcome by modifying the 3D reconstruction scheme. This approach enabled us to ascertain that the three antibodies bind to the same region, to obtain a 3D reconstruction of RyR1 with the antibody bound, and to map SPRY2 to the periphery of the cytoplasmic domain of RyR1. We report here the first 3D localization of a SPRY2 domain in any known RyR isoform.
Conflict of interest statement
Figures


References
-
- Sencer S, Papineni RV, Halling DB, Pate P, Krol J, et al. Coupling of RYR1 and L-type calcium channels via calmodulin binding domains. J Biol Chem. 2001;276:38237–38241. - PubMed
-
- Samso M, Wagenknecht T. Apocalmodulin and Ca2+-calmodulin bind to neighboring locations on the ryanodine receptor. J Biol Chem. 2002;277:1349–1353. - PubMed
-
- Samso M, Shen X, Allen PD. Structural characterization of the RyR1-FKBP12 interaction. J Mol Biol. 2006;356:917–927. - PubMed
-
- Wagenknecht T, Radermacher M, Grassucci R, Berkowitz J, Xin HB, et al. Locations of calmodulin and FK506-binding protein on the three-dimensional architecture of the skeletal muscle ryanodine receptor. J Biol Chem. 1997;272:32463–32471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

