Characteristics of HIV-1 serodiscordant couples enrolled in a clinical trial of antiretroviral pre-exposure prophylaxis for HIV-1 prevention
- PMID: 21998703
- PMCID: PMC3187805
- DOI: 10.1371/journal.pone.0025828
Characteristics of HIV-1 serodiscordant couples enrolled in a clinical trial of antiretroviral pre-exposure prophylaxis for HIV-1 prevention
Abstract
Introduction: Stable heterosexual HIV-1 serodiscordant couples in Africa have high HIV-1 transmission rates and are a critical population for evaluation of new HIV-1 prevention strategies. The Partners PrEP Study is a randomized, double-blind, placebo-controlled trial of tenofovir and emtricitabine-tenofovir pre-exposure prophylaxis to decrease HIV-1 acquisition within heterosexual HIV-1 serodiscordant couples. We describe the trial design and characteristics of the study cohort.
Methods: HIV-1 serodiscordant couples, in which the HIV-1 infected partner did not meet national guidelines for initiation of antiretroviral therapy, were enrolled at 9 research sites in Kenya and Uganda. The HIV-1 susceptible partner was randomized to daily oral tenofovir, emtricitabine-tenofovir, or matching placebo with monthly follow-up for 24-36 months.
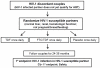
Results: From July 2008 to November 2010, 7920 HIV-1 serodiscordant couples were screened and 4758 enrolled. For 62% (2966/4758) of enrolled couples, the HIV-1 susceptible partner was male. Median age was 33 years for HIV-1 susceptible and HIV-1 infected partners [IQR (28-40) and (26-39) respectively]. Most couples (98%) were married, with a median duration of partnership of 7.0 years (IQR 3.0-14.0) and recent knowledge of their serodiscordant status [median 0.4 years (IQR 0.1-2.0)]. During the month prior to enrollment, couples reported a median of 4 sex acts (IQR 2-8); 27% reported unprotected sex and 14% of male and 1% of female HIV-1 susceptible partners reported sex with outside partners. Among HIV-1 infected partners, the median plasma HIV-1 level was 3.94 log(10) copies/mL (IQR 3.31-4.53) and median CD4 count was 496 cells/µL (IQR 375-662); the majority (64%) had WHO stage 1 HIV-1 disease.
Conclusions: Couples at high risk of HIV-1 transmission were rapidly recruited into the Partners PrEP Study, the largest efficacy trial of oral PrEP. (ClinicalTrials.gov NCT00557245).
Conflict of interest statement
Similar articles
-
Antiretroviral prophylaxis for HIV prevention in heterosexual men and women.N Engl J Med. 2012 Aug 2;367(5):399-410. doi: 10.1056/NEJMoa1108524. Epub 2012 Jul 11. N Engl J Med. 2012. PMID: 22784037 Free PMC article. Clinical Trial.
-
Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa.PLoS Med. 2013;10(9):e1001511. doi: 10.1371/journal.pmed.1001511. Epub 2013 Sep 10. PLoS Med. 2013. PMID: 24058300 Free PMC article. Clinical Trial.
-
What's love got to do with it? Explaining adherence to oral antiretroviral pre-exposure prophylaxis for HIV-serodiscordant couples.J Acquir Immune Defic Syndr. 2012 Apr 15;59(5):463-8. doi: 10.1097/QAI.0b013e31824a060b. J Acquir Immune Defic Syndr. 2012. PMID: 22267018 Free PMC article. Clinical Trial.
-
Emtricitabine/tenofovir disoproxil fumarate: a review of its use in HIV-1 pre-exposure prophylaxis.Drugs. 2013 Mar;73(3):279-91. doi: 10.1007/s40265-013-0024-4. Drugs. 2013. PMID: 23444256 Review.
-
What primary care providers need to know about preexposure prophylaxis for HIV prevention: a narrative review.Ann Intern Med. 2012 Oct 2;157(7):490-7. doi: 10.7326/0003-4819-157-7-201210020-00510. Ann Intern Med. 2012. PMID: 22821365 Free PMC article. Review.
Cited by
-
"I never thought that it would happen … " Experiences of HIV seroconverters among HIV-discordant partnerships in a prospective HIV prevention study in Kenya.AIDS Care. 2016 Dec;28(12):1586-1589. doi: 10.1080/09540121.2016.1191610. Epub 2016 Jun 5. AIDS Care. 2016. PMID: 27264119 Free PMC article.
-
High medication adherence during periconception periods among HIV-1-uninfected women participating in a clinical trial of antiretroviral pre-exposure prophylaxis.J Acquir Immune Defic Syndr. 2014 Sep 1;67(1):91-7. doi: 10.1097/QAI.0000000000000246. J Acquir Immune Defic Syndr. 2014. PMID: 25118795 Free PMC article. Clinical Trial.
-
Unreported antiretroviral use by HIV-1-infected participants enrolling in a prospective research study.J Acquir Immune Defic Syndr. 2014 Feb 1;65(2):e90-4. doi: 10.1097/QAI.0b013e3182a2db02. J Acquir Immune Defic Syndr. 2014. PMID: 24442233 Free PMC article. Clinical Trial. No abstract available.
-
HIV Transmission Risk Persists During the First 6 Months of Antiretroviral Therapy.J Acquir Immune Defic Syndr. 2016 Aug 15;72(5):579-84. doi: 10.1097/QAI.0000000000001019. J Acquir Immune Defic Syndr. 2016. PMID: 27070123 Free PMC article.
-
Antiretroviral prophylaxis for HIV prevention in heterosexual men and women.N Engl J Med. 2012 Aug 2;367(5):399-410. doi: 10.1056/NEJMoa1108524. Epub 2012 Jul 11. N Engl J Med. 2012. PMID: 22784037 Free PMC article. Clinical Trial.
References
-
- AVAC website. Oral and Topical PrEP Trials Timeline. Available from: www.avac.org/prep. Accessed 2011 March 23.
-
- Tsai CC, Follis KE, Sabo A, Beck TW, Grant RF, et al. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. - PubMed
-
- Garcia-Lerma JG, Cong ME, Mitchell J, Youngpairoj AS, Zheng Q, et al. Intermittent prophylaxis with oral truvada protects macaques from rectal SHIV infection. Science Translational Medicine. 2010;2:14ra14. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous