Direct translocation as major cellular uptake for CADY self-assembling peptide-based nanoparticles
- PMID: 21998722
- PMCID: PMC3187819
- DOI: 10.1371/journal.pone.0025924
Direct translocation as major cellular uptake for CADY self-assembling peptide-based nanoparticles
Abstract
Cell penetrating peptides constitute a potent approach to overcome the limitations of in vivo siRNA delivery. We recently proposed a peptide-based nanoparticle system, CADY, for efficient delivery of siRNA into numerous cell lines. CADY is a secondary amphipathic peptide that forms stable complexes with siRNA thereby improving both their cellular uptake and biological response. With the aim of understanding the cellular uptake mechanism of CADY:siRNA complexes, we have combined biochemical, confocal and electron microscopy approaches. In the present work, we provide evidence that the major route for CADY:siRNA cellular uptake involves direct translocation through the membrane but not the endosomal pathway. We have demonstrated that CADY:siRNA complexes do not colocalize with most endosomal markers and remain fully active in the presence of inhibitors of the endosomal pathway. Moreover, neither electrostatic interactions with cell surface heparan sulphates nor membrane potential are essential for CADY:siRNA cell entry. In contrast, we have shown that CADY:siRNA complexes clearly induce a transient cell membrane permeabilization, which is rapidly restored by cell membrane fluidity. Therefore, we propose that direct translocation is the major gate for cell entry of CADY:siRNA complexes. Membrane perturbation and uptake are driven mainly by the ability of CADY to interact with phospholipids within the cell membrane, followed by rapid localization of the complex in the cytoplasm, without affecting cell integrity or viability.
Conflict of interest statement
Figures
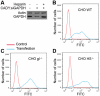
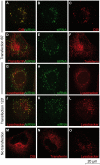
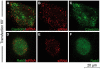

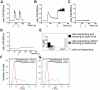

References
-
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1988;391:806–811. - PubMed
-
- Dorsett Y, Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nat Rev Drug Discovery. 2004;3:318–329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

