An angiotensin I-converting enzyme mutation (Y465D) causes a dramatic increase in blood ACE via accelerated ACE shedding
- PMID: 21998728
- PMCID: PMC3187827
- DOI: 10.1371/journal.pone.0025952
An angiotensin I-converting enzyme mutation (Y465D) causes a dramatic increase in blood ACE via accelerated ACE shedding
Abstract
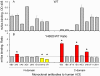
Background: Angiotensin I-converting enzyme (ACE) metabolizes a range of peptidic substrates and plays a key role in blood pressure regulation and vascular remodeling. Thus, elevated ACE levels may be associated with an increased risk for different cardiovascular or respiratory diseases. Previously, a striking familial elevation in blood ACE was explained by mutations in the ACE juxtamembrane region that enhanced the cleavage-secretion process. Recently, we found a family whose affected members had a 6-fold increase in blood ACE and a Tyr465Asp (Y465D) substitution, distal to the stalk region, in the N domain of ACE.
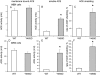
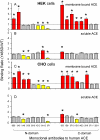
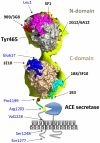
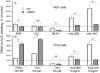
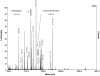
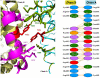
Methodology/principal findings: HEK and CHO cells expressing mutant (Tyr465Asp) ACE demonstrate a 3- and 8-fold increase, respectively, in the rate of ACE shedding compared to wild-type ACE. Conformational fingerprinting of mutant ACE demonstrated dramatic changes in ACE conformation in several different epitopes of ACE. Cell ELISA carried out on CHO-ACE cells also demonstrated significant changes in local ACE conformation, particularly proximal to the stalk region. However, the cleavage site of the mutant ACE--between Arg1203 and Ser1204--was the same as that of WT ACE. The Y465D substitution is localized in the interface of the N-domain dimer (from the crystal structure) and abolishes a hydrogen bond between Tyr465 in one monomer and Asp462 in another.
Conclusions/significance: The Y465D substitution results in dramatic increase in the rate of ACE shedding and is associated with significant local conformational changes in ACE. These changes could result in increased ACE dimerization and accessibility of the stalk region or the entire sACE, thus increasing the rate of cleavage by the putative ACE secretase (sheddase).
Conflict of interest statement
Figures



References
-
- Ehlers MWR, Riordan JF. Angiotensin-converting enzyme: new concepts concerning its biological role. Biochemistry. 1989;8:5311–5318. - PubMed
-
- Skidgel RA, Erdos EG. Biochemistry of angiotensin I- converting enzyme. In: Robertson JIS, Nichols MG, editors. The Renin-Angiotensin System. Glower Medical Publishing, New York; 1993. pp. 10.1–10.10.
-
- Corvol P, Eyries M, Soubrier F. Peptidyl-dipeptidase A/Angiotensin I-converting enzyme. In: Barret A, Rawlings N, Woessner J, editors. Handbook of Proteolytic Enzymes. Elsevier Academic Press. New York; 2004. pp. 332–349.
-
- Bernstein KE, Xiao HD, Frenzel K, Li P, Shen XZ, et al. Six truisms concerning ACE and the renin-angiotensin system educed from the genetic analysis of mice. Circ Res. 2005;96:1135–1144. - PubMed
-
- Caldwell PR, Seegal BC, Hsu KC, Das H, Soffer RL. Angiotensin-converting enztme: vascular endothelial localization. Science. 1976;191:1050–1051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

