TLR1/TLR2 heterodimers play an important role in the recognition of Borrelia spirochetes
- PMID: 21998742
- PMCID: PMC3187844
- DOI: 10.1371/journal.pone.0025998
TLR1/TLR2 heterodimers play an important role in the recognition of Borrelia spirochetes
Abstract
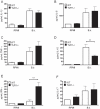
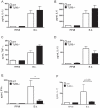
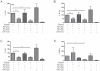
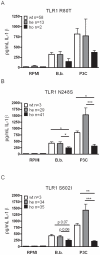
After infection with Borrelia species, the risk for developing Lyme disease varies significantly between individuals. Recognition of Borrelia by the immune system is mediated by pattern recognition receptors (PRRs), such as TLRs. While TLR2 is the main recognition receptor for Borrelia spp., little is known about the role of TLR1 and TLR6, which both can form functionally active heterodimers with TLR2. Here we investigated the recognition of Borrelia by both murine and human TLR1 and TLR6. Peritoneal macrophages from TLR1- and TLR6- gene deficient mice were isolated and exposed to Borrelia. Human PBMCs were stimulated with Borrelia with or without specific TLR1 and TLR6 blocking using specific antibodies. Finally, the functional consequences of TLR polymorphisms on Borrelia-induced cytokine production were assessed. Splenocytes isolated from both TLR1-/- and TLR6-/- mice displayed a distorted Th1/Th2 cytokine balance after stimulation with B.burgdorferi, while no differences in pro-inflammatory cytokine production were observed. In contrast, blockade of TLR1 with specific neutralizing antibodies led to decreased cytokine production by human PBMCs after exposure to B.burgdorferi. Blockade of human TLR6 did not lead to suppression of cytokine production. When PBMCs from healthy individuals bearing polymorphisms in TLR1 were exposed to B.burgdorferi, a remarkably decreased in vitro cytokine production was observed in comparison to wild-type controls. TLR6 polymorphisms lead to a minor modified cytokine production. This study indicates a dominant role for TLR1/TLR2 heterodimers in the induction of the early inflammatory response by Borrelia spirochetes in humans.
Conflict of interest statement
Figures

References
-
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, et al. Lyme disease-a tick-borne spirochetosis? Science. 1982;216:1317. - PubMed
-
- Balmelli T, Piffaretti JC. Association between different clinical manifestations of Lyme disease and different species of Borrelia burgdorferi sensu lato. . Res Microbiol. 1995;146:329. - PubMed
-
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

