Apolipoprotein J/Clusterin is a novel structural component of human erythrocytes and a biomarker of cellular stress and senescence
- PMID: 21998749
- PMCID: PMC3188580
- DOI: 10.1371/journal.pone.0026032
Apolipoprotein J/Clusterin is a novel structural component of human erythrocytes and a biomarker of cellular stress and senescence
Abstract
Background: Secretory Apolipoprotein J/Clusterin (sCLU) is a ubiquitously expressed chaperone that has been functionally implicated in several pathological conditions of increased oxidative injury, including aging. Nevertheless, the biological role of sCLU in red blood cells (RBCs) remained largely unknown. In the current study we identified sCLU as a component of human RBCs and we undertook a detailed analysis of its cellular topology. Moreover, we studied the erythrocytic membrane sCLU content during organismal aging, in conditions of increased organismal stress and accelerated RBCs senescence, as well as during physiological in vivo cellular senescence.
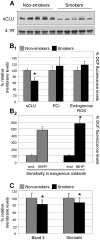
Methodology/principal findings: By using a combination of molecular, biochemical and high resolution microscopical methods we found that sCLU is a novel structural component of RBCs extra- and intracellular plasma membrane and cytosol. We observed that the RBCs membrane-associated sCLU decreases during organismal aging or exposure to acute stress (e.g. smoking), in patients with congenital hemolytic anemia, as well as during RBCs in vivo senescence. In all cases, sCLU reduction paralleled the expression of typical cellular senescence, redox imbalance and erythrophagocytosis markers which are also indicative of the senescence- and oxidative stress-mediated RBCs membrane vesiculation.
Conclusions/significance: We propose that sCLU at the mature RBCs is not a silent remnant of the erythroid precursors, but an active component being functionally implicated in the signalling mechanisms of cellular senescence and oxidative stress-responses in both healthy and diseased organism. The reduced sCLU protein levels in the RBCs membrane following cell exposure to various endogenous or exogenous stressors closely correlates to the levels of cellular senescence and redox imbalance markers, suggesting the usefulness of sCLU as a sensitive biomarker of senescence and cellular stress.
Conflict of interest statement
Figures





References
-
- Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. NO inhibits signal transduction pathway for ATP release from erythrocytes via its action on heterotrimeric G protein Gi. Am J Physiol Heart Circ Physiol. 2004;287:H748–754. - PubMed
-
- Bosman GJ, Werre JM, Willekens FL, Novotny VM. Erythrocyte aging in vivo and in vitro: structural aspects and implications for transfusion. Transfus Med. 2008;18:335–347. - PubMed
-
- Mohandas N, Groner W. Cell membrane and volume changes during red cell development and aging. Ann N Y Acad Sci. 1989;554:217–224. - PubMed
-
- Kay M. Immunoregulation of cellular life span. Ann N Y Acad Sci. 2005;1057:85–111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

