Protective effect of tetrahydroxystilbene glucoside on 6-OHDA-induced apoptosis in PC12 cells through the ROS-NO pathway
- PMID: 21998750
- PMCID: PMC3188584
- DOI: 10.1371/journal.pone.0026055
Protective effect of tetrahydroxystilbene glucoside on 6-OHDA-induced apoptosis in PC12 cells through the ROS-NO pathway
Abstract
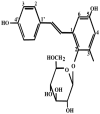
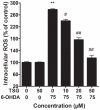
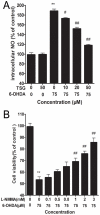
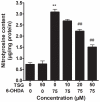
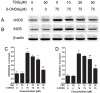
Oxidative stress plays an important role in the pathogenesis of neurodegenerative diseases, such as Parkinson's disease. The molecule, 2,3,5,4'-tetrahydr- oxystilbene-2-O-β-D-glucoside (TSG), is a potent antioxidant derived from the Chinese herb, Polygonum multiflorum Thunb. In this study, we investigated the protective effect of TSG against 6-hydroxydopamine-induced apoptosis in rat adrenal pheochromocytoma PC12 cells and the possible mechanisms. Our data demonstrated that TSG significantly reversed the 6-hydroxydopamine-induced decrease in cell viability, prevented 6-hydroxydopamine-induced changes in condensed nuclei and decreased the percentage of apoptotic cells in a dose-dependent manner. In addition, TSG slowed the accumulation of intracellular reactive oxygen species and nitric oxide, counteracted the overexpression of inducible nitric oxide syntheses as well as neuronal nitric oxide syntheses, and also reduced the level of protein-bound 3-nitrotyrosine. These results demonstrate that the protective effects of TSG on rat adrenal pheochromocytoma PC12 cells are mediated, at least in part, by the ROS-NO pathway. Our results indicate that TSG may be effective in providing protection against neurodegenerative diseases associated with oxidative stress.
Conflict of interest statement
Figures



Similar articles
-
Protective effects of Lycium barbarum polysaccharide on 6-OHDA-induced apoptosis in PC12 cells through the ROS-NO pathway.Molecules. 2014 Dec 24;20(1):293-308. doi: 10.3390/molecules20010293. Molecules. 2014. PMID: 25547727 Free PMC article.
-
Protective effects of 2,3,5,4'-tetrahydroxystilbene-2-O-β-D-glucoside in the MPTP-induced mouse model of Parkinson's disease: Involvement of reactive oxygen species-mediated JNK, P38 and mitochondrial pathways.Eur J Pharmacol. 2015 Nov 15;767:175-82. doi: 10.1016/j.ejphar.2015.10.023. Epub 2015 Oct 20. Eur J Pharmacol. 2015. PMID: 26477638
-
The Protective Effect of Tetrahydroxystilbene Glucoside on High Glucose-Induced Injury in Human Umbilical Vein Endothelial Cells through the PI3K/Akt/eNOS Pathway and Regulation of Bcl-2/Bax.J Vasc Res. 2021;58(5):301-310. doi: 10.1159/000511035. Epub 2021 Jul 2. J Vasc Res. 2021. PMID: 34218226
-
Biological Effects of Tetrahydroxystilbene Glucoside: An Active Component of a Rhizome Extracted from Polygonum multiflorum.Oxid Med Cell Longev. 2018 Nov 4;2018:3641960. doi: 10.1155/2018/3641960. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30524653 Free PMC article. Review.
-
Current pharmacological developments in 2,3,4',5-tetrahydroxystilbene 2-O-β-D-glucoside (TSG).Eur J Pharmacol. 2017 Sep 15;811:21-29. doi: 10.1016/j.ejphar.2017.05.037. Epub 2017 May 23. Eur J Pharmacol. 2017. PMID: 28545778 Review.
Cited by
-
The Neuro-Protective Effects of the TSPO Ligands CB86 and CB204 on 6-OHDA-Induced PC12 Cell Death as an In Vitro Model for Parkinson's Disease.Biology (Basel). 2021 Nov 15;10(11):1183. doi: 10.3390/biology10111183. Biology (Basel). 2021. PMID: 34827176 Free PMC article.
-
Herb-Induced Liver Injury Related to Reynoutria multiflora (Thunb.) Moldenke: Risk Factors, Molecular and Mechanistic Specifics.Front Pharmacol. 2021 Sep 2;12:738577. doi: 10.3389/fphar.2021.738577. eCollection 2021. Front Pharmacol. 2021. PMID: 34539416 Free PMC article. Review.
-
Resveratrol protects PC12 cells against OGD/ R-induced apoptosis via the mitochondrial-mediated signaling pathway.Acta Biochim Biophys Sin (Shanghai). 2016 Apr;48(4):342-53. doi: 10.1093/abbs/gmw011. Acta Biochim Biophys Sin (Shanghai). 2016. PMID: 26960953 Free PMC article.
-
Botanical Drug Puerarin Attenuates 6-Hydroxydopamine (6-OHDA)-Induced Neurotoxicity via Upregulating Mitochondrial Enzyme Arginase-2.Mol Neurobiol. 2016 May;53(4):2200-11. doi: 10.1007/s12035-015-9195-1. Epub 2015 May 8. Mol Neurobiol. 2016. PMID: 25952544
-
Rationale and design of the Helping Ease Renal failure with Bupi Yishen compared with the Angiotensin II Antagonist Losartan (HERBAAL) trial: a randomized controlled trial in non-diabetes stage 4 chronic kidney disease.BMC Complement Altern Med. 2015 Sep 8;15:316. doi: 10.1186/s12906-015-0830-1. BMC Complement Altern Med. 2015. PMID: 26351087 Free PMC article. Clinical Trial.
References
-
- de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5:525–535. - PubMed
-
- Hornykiewicz O, Kish SJ. Biochemical pathophysiology of Parkinson's disease. Adv Neurol. 1987;45:19–34. - PubMed
-
- Jellinger KA, Kienzl E, Rumpelmaier G, Paulus W, Riederer P, et al. Iron and ferritin in substantia nigra in Parkinson's disease. Adv Neurol. 1993;60:267–272. - PubMed
-
- Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, et al. Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36:348–355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

