A new baseline for fascioliasis in Venezuela: lymnaeid vectors ascertained by DNA sequencing and analysis of their relationships with human and animal infection
- PMID: 21999170
- PMCID: PMC3213164
- DOI: 10.1186/1756-3305-4-200
A new baseline for fascioliasis in Venezuela: lymnaeid vectors ascertained by DNA sequencing and analysis of their relationships with human and animal infection
Abstract
Background: Human and animal fascioliasis poses serious public health problems in South America. In Venezuela, livestock infection represents an important veterinary problem whereas there appear to be few human cases reported, most of which are passively detected in health centres. However, results of recent surveys suggest that the situation may be underestimated in particular areas. To obtain a baseline for future fascioliasis assessment, studies were undertaken by means of rDNA ITS-2 and ITS-1 and mtDNA cox1 sequencing to clarify the specific status of Venezuelan lymnaeids, their geographical distribution and fascioliasis transmission capacity, by comparison with other American countries and other continents.
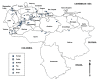
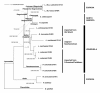
Results: Results obtained completely change the lymnaeid scenario known so far. The relatively rich lymnaeid fauna of Venezuela has been proven to include (i) Lymnaea meridensis and L. neotropica as the only native members, (ii) L. cubensis and Pseudosuccinea columella introduced from the Caribbean area, and (iii) Galba truncatula and L. schirazensis introduced from the Old World. The absence of representatives of the stagnicoline and Radix groups is remarkable. Four species are fascioliasis vectors: G. truncatula, L. cubensis and L. neotropica, which have the capacity to give rise to human endemic areas, and P. columella, which is a source of animal infection and is responsible for the spread of disease. Vector capacity in the apparently highland endemic L. meridensis is to be confimed, although may be expected given its phylogenetic relationships. Similarly as elsewhere, the non-transmitting L. schirazensis has been confused with L. cubensis, also with G. truncatula and possibly with L. neotropica.
Conclusions: The new scenario leads to the re-opening of many disease aspects. In Venezuela, altitude appears to be the main factor influencing fascioliasis distribution. Human infection shows an altitude pattern similar to other Andean countries, although a differing highland/lowland impact on animal infection does not appear evident. The overlap of G. truncatula, L. cubensis and probably also L. neotropica in temperate and cold zones suggests a higher risk for human infection in mid and high altitude areas. A lymnaeid species mapping by means of DNA markers becomes a priority to determine human and animal fascioliasis distribution in Venezuela, owing to the importance of lymnaeid vectors in defining transmission and epidemiological patterns.
Figures





Similar articles
-
DNA multigene characterization of Fasciola hepatica and Lymnaea neotropica and its fascioliasis transmission capacity in Uruguay, with historical correlation, human report review and infection risk analysis.PLoS Negl Trop Dis. 2017 Feb 3;11(2):e0005352. doi: 10.1371/journal.pntd.0005352. eCollection 2017 Feb. PLoS Negl Trop Dis. 2017. PMID: 28158188 Free PMC article.
-
Molecular characterisation of Galba truncatula, Lymnaea neotropica and L. schirazensis from Cajamarca, Peru and their potential role in transmission of human and animal fascioliasis.Parasit Vectors. 2012 Aug 15;5:174. doi: 10.1186/1756-3305-5-174. Parasit Vectors. 2012. PMID: 22894178 Free PMC article.
-
DNA sequence characterisation and phylogeography of Lymnaea cousini and related species, vectors of fascioliasis in northern Andean countries, with description of L. meridensis n. sp. (Gastropoda: Lymnaeidae).Parasit Vectors. 2011 Jul 12;4:132. doi: 10.1186/1756-3305-4-132. Parasit Vectors. 2011. PMID: 21749718 Free PMC article.
-
Chapter 2. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control.Adv Parasitol. 2009;69:41-146. doi: 10.1016/S0065-308X(09)69002-3. Adv Parasitol. 2009. PMID: 19622408 Review.
-
Reviewing lymnaeid vectors of fascioliasis by ribosomal DNA sequence analyses.J Helminthol. 2005 Sep;79(3):257-67. doi: 10.1079/joh2005297. J Helminthol. 2005. PMID: 16153320 Review.
Cited by
-
DNA multigene characterization of Fasciola hepatica and Lymnaea neotropica and its fascioliasis transmission capacity in Uruguay, with historical correlation, human report review and infection risk analysis.PLoS Negl Trop Dis. 2017 Feb 3;11(2):e0005352. doi: 10.1371/journal.pntd.0005352. eCollection 2017 Feb. PLoS Negl Trop Dis. 2017. PMID: 28158188 Free PMC article.
-
Spread of the fascioliasis endemic area assessed by seasonal follow-up of rDNA ITS-2 sequenced lymnaeid populations in Cajamarca, Peru.One Health. 2021 May 11;13:100265. doi: 10.1016/j.onehlt.2021.100265. eCollection 2021 Dec. One Health. 2021. PMID: 34041348 Free PMC article.
-
Human and Animal Fascioliasis: Origins and Worldwide Evolving Scenario.Clin Microbiol Rev. 2022 Dec 21;35(4):e0008819. doi: 10.1128/cmr.00088-19. Epub 2022 Dec 5. Clin Microbiol Rev. 2022. PMID: 36468877 Free PMC article. Review.
-
One Health Action against Human Fascioliasis in the Bolivian Altiplano: Food, Water, Housing, Behavioural Traditions, Social Aspects, and Livestock Management Linked to Disease Transmission and Infection Sources.Int J Environ Res Public Health. 2022 Jan 20;19(3):1120. doi: 10.3390/ijerph19031120. Int J Environ Res Public Health. 2022. PMID: 35162146 Free PMC article.
-
Sheep and Cattle Reservoirs in the Highest Human Fascioliasis Hyperendemic Area: Experimental Transmission Capacity, Field Epidemiology, and Control Within a One Health Initiative in Bolivia.Front Vet Sci. 2020 Oct 27;7:583204. doi: 10.3389/fvets.2020.583204. eCollection 2020. Front Vet Sci. 2020. PMID: 33195605 Free PMC article.
References
-
- Mas-Coma S, Valero MA, Bargues MD. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv Parasitol. 2009;69:41–146. - PubMed
-
- World Health Organization. Control of foodborne trematode infections. WHO Techn Rep Ser. 1995;849:1–157. - PubMed
-
- Mas-Coma S, Valero MA, Bargues MD. Effects of climate change on animal and zoonotic helminthiases. Rev Sci Techn Off Int Epiz. 2008;27:443–457. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

