The cooperative action of bacterial fibronectin-binding proteins and secreted proteins promote maximal Campylobacter jejuni invasion of host cells by stimulating membrane ruffling
- PMID: 21999233
- PMCID: PMC3262099
- DOI: 10.1111/j.1462-5822.2011.01714.x
The cooperative action of bacterial fibronectin-binding proteins and secreted proteins promote maximal Campylobacter jejuni invasion of host cells by stimulating membrane ruffling
Abstract
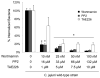
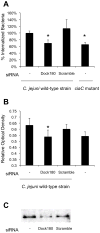
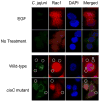
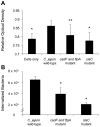
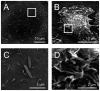
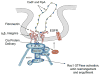
This study was performed to elucidate the host cell scaffolding and signalling molecules that Campylobacter jejuni utilizes to invade epithelial cells. We hypothesized that the C. jejuni fibronectin-binding proteins and secreted proteins are required for cell signalling and maximal invasion of host cells. C. jejuni binding to host cells via the CadF and FlpA fibronectin-binding proteins activated the epidermal growth factor (EGF) pathway, as evidenced by inhibitor studies and immunoprecipitation coupled with immunoblot analysis using antibodies reactive against total and active EGF receptor. Inhibitor studies revealed maximal C. jejuni host cell invasion was dependent upon PI3-Kinase, c-Src and focal adhesion kinase (FAK), all of which are known to participate in cytoskeletal rearrangements. Knockdown of endogenous Dock180, which is a Rac1-specific guanine nucleotide exchange factor, using siRNA revealed that C. jejuni invasion was significantly reduced compared with cells treated with scrambled siRNA. We further demonstrated that the C. jejuni Cia proteins are, in part, responsible for Rho GTPase Rac1 recruitment and activation, as judged by immunofluorescence microscopy and Rac1 activation. Based on these data, we present a model that illustrates that C. jejuni utilizes a coordinated mechanism involving both adhesins and secreted proteins to promote membrane ruffling and host cell invasion.
© 2011 Blackwell Publishing Ltd.
Figures

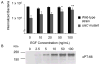
References
-
- Beardsley A, Fang K, Mertz H, Castranova V, Friend S, Liu J. Loss of caveolin-1 polarity impedes endothelial cell polarization and directional movement. J Biol Chem. 2005;280:3541–3547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

