Growth independent rhamnolipid production from glucose using the non-pathogenic Pseudomonas putida KT2440
- PMID: 21999513
- PMCID: PMC3258213
- DOI: 10.1186/1475-2859-10-80
Growth independent rhamnolipid production from glucose using the non-pathogenic Pseudomonas putida KT2440
Abstract
Background: Rhamnolipids are potent biosurfactants with high potential for industrial applications. However, rhamnolipids are currently produced with the opportunistic pathogen Pseudomonas aeruginosa during growth on hydrophobic substrates such as plant oils. The heterologous production of rhamnolipids entails two essential advantages: Disconnecting the rhamnolipid biosynthesis from the complex quorum sensing regulation and the opportunity of avoiding pathogenic production strains, in particular P. aeruginosa. In addition, separation of rhamnolipids from fatty acids is difficult and hence costly.
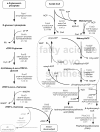
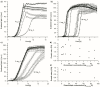
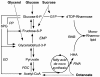
Results: Here, the metabolic engineering of a rhamnolipid producing Pseudomonas putida KT2440, a strain certified as safety strain using glucose as carbon source to avoid cumbersome product purification, is reported. Notably, P. putida KT2440 features almost no changes in growth rate and lag-phase in the presence of high concentrations of rhamnolipids (> 90 g/L) in contrast to the industrially important bacteria Bacillus subtilis, Corynebacterium glutamicum, and Escherichia coli. P. putida KT2440 expressing the rhlAB-genes from P. aeruginosa PAO1 produces mono-rhamnolipids of P. aeruginosa PAO1 type (mainly C(10):C(10)). The metabolic network was optimized in silico for rhamnolipid synthesis from glucose. In addition, a first genetic optimization, the removal of polyhydroxyalkanoate formation as competing pathway, was implemented. The final strain had production rates in the range of P. aeruginosa PAO1 at yields of about 0.15 g/g(glucose) corresponding to 32% of the theoretical optimum. What's more, rhamnolipid production was independent from biomass formation, a trait that can be exploited for high rhamnolipid production without high biomass formation.
Conclusions: A functional alternative to the pathogenic rhamnolipid producer P. aeruginosa was constructed and characterized. P. putida KT24C1 pVLT31_rhlAB featured the highest yield and titer reported from heterologous rhamnolipid producers with glucose as carbon source. Notably, rhamnolipid production was uncoupled from biomass formation, which allows optimal distribution of resources towards rhamnolipid synthesis. The results are discussed in the context of rational strain engineering by using the concepts of synthetic biology like chassis cells and orthogonality, thereby avoiding the complex regulatory programs of rhamnolipid production existing in the natural producer P. aeruginosa.
Figures
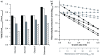
Similar articles
-
Heterologous Rhamnolipid Biosynthesis: Advantages, Challenges, and the Opportunity to Produce Tailor-Made Rhamnolipids.Front Bioeng Biotechnol. 2020 Oct 22;8:594010. doi: 10.3389/fbioe.2020.594010. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33195161 Free PMC article. Review.
-
Biofilm as a production platform for heterologous production of rhamnolipids by the non-pathogenic strain Pseudomonas putida KT2440.Microb Cell Fact. 2016 Oct 24;15(1):181. doi: 10.1186/s12934-016-0581-9. Microb Cell Fact. 2016. PMID: 27776509 Free PMC article.
-
Heterologous production of long-chain rhamnolipids from Burkholderia glumae in Pseudomonas putida-a step forward to tailor-made rhamnolipids.Appl Microbiol Biotechnol. 2018 Feb;102(3):1229-1239. doi: 10.1007/s00253-017-8702-x. Epub 2017 Dec 20. Appl Microbiol Biotechnol. 2018. PMID: 29264775
-
Designer rhamnolipids by reduction of congener diversity: production and characterization.Microb Cell Fact. 2017 Dec 14;16(1):225. doi: 10.1186/s12934-017-0838-y. Microb Cell Fact. 2017. PMID: 29241456 Free PMC article.
-
Rhamnolipids--next generation surfactants?J Biotechnol. 2012 Dec 31;162(4):366-80. doi: 10.1016/j.jbiotec.2012.05.022. Epub 2012 Jun 20. J Biotechnol. 2012. PMID: 22728388 Review.
Cited by
-
Surface sensing triggers a broad-spectrum antimicrobial response in Pseudomonas aeruginosa.Environ Microbiol. 2020 Aug;22(8):3572-3587. doi: 10.1111/1462-2920.15139. Epub 2020 Jul 10. Environ Microbiol. 2020. PMID: 32573899 Free PMC article.
-
Biosurfactants and Synthetic Surfactants in Bioelectrochemical Systems: A Mini-Review.Front Microbiol. 2020 Mar 13;11:358. doi: 10.3389/fmicb.2020.00358. eCollection 2020. Front Microbiol. 2020. PMID: 32231644 Free PMC article. Review.
-
Integrated foam fractionation for heterologous rhamnolipid production with recombinant Pseudomonas putida in a bioreactor.AMB Express. 2016 Mar;6(1):11. doi: 10.1186/s13568-016-0183-2. Epub 2016 Feb 9. AMB Express. 2016. PMID: 26860613 Free PMC article.
-
Heterologous Rhamnolipid Biosynthesis: Advantages, Challenges, and the Opportunity to Produce Tailor-Made Rhamnolipids.Front Bioeng Biotechnol. 2020 Oct 22;8:594010. doi: 10.3389/fbioe.2020.594010. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33195161 Free PMC article. Review.
-
Bioprocess exploitation of microaerobic auto-induction using the example of rhamnolipid biosynthesis in Pseudomonas putida KT2440.J Biol Eng. 2025 Jan 18;19(1):8. doi: 10.1186/s13036-025-00478-z. J Biol Eng. 2025. PMID: 39827133 Free PMC article.
References
-
- Gautam KK, Tyagi VK. Microbial surfactants: A review. J Oleo Sci. 2006;55:155–166. doi: 10.5650/jos.55.155. - DOI
-
- Rahman PKSM, Gakpe E. Production, characterisation and applications of biosurfactants - Review. Biotechnology. 2008;7:360–370. doi: 10.3923/biotech.2008.360.370. - DOI
-
- Abalos A, Pinazo A, Infante MR, Casals M, García F, Manresa A. Physicochemical and antimicrobial properties of new rhamnolipids produced by Pseudomonas aeruginosa AT10 from soybean oil refinery wastes. Langmuir. 2001;17:1367–1371. doi: 10.1021/la0011735. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

