Bacterial transition metal P(1B)-ATPases: transport mechanism and roles in virulence
- PMID: 21999638
- PMCID: PMC3224801
- DOI: 10.1021/bi201418k
Bacterial transition metal P(1B)-ATPases: transport mechanism and roles in virulence
Abstract
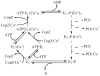
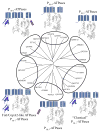
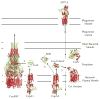
P(1B)-type ATPases are polytopic membrane proteins that couple the hydrolysis of ATP to the efflux of cytoplasmic transition metals. This paper reviews recent progress in our understanding of the structure and function of these proteins in bacteria. These are members of the P-type superfamily of transport ATPases. Cu(+)-ATPases are the most frequently observed and best-characterized members of this group of transporters. However, bacterial genomes show diverse arrays of P(1B)-type ATPases with a range of substrates (Cu(+), Zn(2+), Co(2+)). Furthermore, because of the structural similarities among transitions metals, these proteins can also transport nonphysiological substrates (Cd(2+), Pb(2+), Au(+), Ag(+)). P(1B)-type ATPases have six or eight transmembrane segments (TM) with metal coordinating amino acids in three core TMs flanking the cytoplasmic domain responsible for ATP binding and hydrolysis. In addition, regulatory cytoplasmic metal binding domains are present in most P(1B)-type ATPases. Central to the transport mechanism is the binding of the uncomplexed metal to these proteins when cytoplasmic substrates are bound to chaperone and chelating molecules. Metal binding to regulatory sites is through a reversible metal exchange among chaperones and cytoplasmic metal binding domains. In contrast, the chaperone-mediated metal delivery to transport sites appears as a largely irreversible event. P(1B)-ATPases have two overarching physiological functions: to maintain cytoplasmic metal levels and to provide metals for the periplasmic assembly of metalloproteins. Recent studies have shown that both roles are critical for bacterial virulence, since P(1B)-ATPases appear key to overcome high phagosomal metal levels and are required for the assembly of periplasmic and secreted metalloproteins that are essential for survival in extreme oxidant environments.
© 2011 American Chemical Society
Figures


References
-
- Fraústro da Silva JJR, Williams RJP. The biological chemistry of the elements. 2. Oxford Unviversity Press; New York: 2001.
-
- Shi W, Zhan C, Ignatov A, Manjasetty BA, Marinkovic N, Sullivan M, Huang R, Chance MR. Metalloproteomics: high-throughput structural and functional annotation of proteins in structural genomics. Structure. 2005;13:1473–1486. - PubMed
-
- Cvetkovic A, Menon AL, Thorgersen MP, Scott JW, Poole FL, 2nd, Jenney FE, Jr, Lancaster WA, Praissman JL, Shanmukh S, Vaccaro BJ, Trauger SA, Kalisiak E, Apon JV, Siuzdak G, Yannone SM, Tainer JA, Adams MW. Microbial metalloproteomes are largely uncharacterized. Nature. 2010;466:779–782. - PubMed
-
- Goldstein S, Meyerstein D, Czapski G. The Fenton reagents. Free Radical Biol Med. 1993;15:435–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

