Smardaesidins A-G, isopimarane and 20-nor-isopimarane diterpenoids from Smardaea sp., a fungal endophyte of the moss Ceratodon purpureus
- PMID: 21999655
- PMCID: PMC3371368
- DOI: 10.1021/np2000864
Smardaesidins A-G, isopimarane and 20-nor-isopimarane diterpenoids from Smardaea sp., a fungal endophyte of the moss Ceratodon purpureus
Abstract
Five new isopimarane diterpenes, smardaesidins A-E (1- 5) and two new 20-nor-isopimarane diterpenes, smardaesidins F (6) and G (7), together with sphaeropsidins A (8) and C-F (10-13) were isolated from an endophytic fungal strain, Smardaea sp. AZ0432, occurring in living photosynthetic tissue of the moss Ceratodon purpureus . Of these, smardaesidins B (2) and C (3) were obtained as an inseparable mixture of isomers. Chemical reduction of sphaeropsidin A (8) afforded sphaeropsidin B (9), whereas catalytic hydrogenation of 8 yielded 7-O-15,16-tetrahydrosphaeropsidin A (14) and its new derivative, 7-hydroxy-6-oxoisopimara-7-en-20-oic acid (15). The acetylation and diazomethane reaction of sphaeropsidin A (8) afforded two of its known derivatives, 6-O-acetylsphaeropsidin A (16) and 8,14-methylenesphaeropsidin A methyl ester (17), respectively. Methylation of 10 yielded sphaeropsidin C methyl ester (18). The planar structures and relative configurations of the new compounds 1-7 and 15 were elucidated using MS and 1D and 2D NMR experiments, while the absolute configurations of the stereocenters of 4 and 6-8 were assigned using a modified Mosher's ester method, CD spectra, and comparison of specific rotation data with literature values. Compounds 1-18 were evaluated for their potential anticancer activity using several cancer cell lines and cells derived from normal human primary fibroblasts. Of these, compounds 8, 11, and 16 showed significant cytotoxic activity. More importantly, sphaeropsidin A (8) showed cell-type selectivity in the cytotoxicity assay and inhibited migration of metastatic breast adenocarcinoma (MDA-MB-231) cells at subcytotoxic concentrations.
Figures

 ) and HMBC correlations (H→C) of 1.
) and HMBC correlations (H→C) of 1.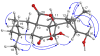

 ) and HMBC correlations (H→C) of 5.
) and HMBC correlations (H→C) of 5.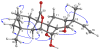
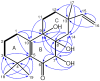
 ) and HMBC correlations (H→C) of 6.
) and HMBC correlations (H→C) of 6.


Similar articles
-
Botrysphones A-C and Botrysphins A-F, Triketides and Diterpenoids from the Fungus Botrysphaeria laricina.J Nat Prod. 2017 Jun 23;80(6):1791-1797. doi: 10.1021/acs.jnatprod.6b01196. Epub 2017 Jun 13. J Nat Prod. 2017. PMID: 28609099
-
20-nor-Isopimarane and isopimarane diterpenoids produced by Aspergillus sp. WT03.Org Biomol Chem. 2023 Mar 1;21(9):1895-1902. doi: 10.1039/d3ob00005b. Org Biomol Chem. 2023. PMID: 36752060
-
A new 3,4-seco-isopimarane and three new isopimarane diterpenoids from Kaempferia champasakensis collected from Vietnam and their cytotoxic activities.J Nat Med. 2024 Jun;78(3):537-546. doi: 10.1007/s11418-024-01789-z. Epub 2024 Mar 22. J Nat Med. 2024. PMID: 38517624
-
Crassolides A-F, cembranoids with a trans-fused lactone from the soft coral Sarcophyton crassocaule.J Nat Prod. 2006 Nov;69(11):1554-9. doi: 10.1021/np060182w. J Nat Prod. 2006. PMID: 17125220
-
Sphaeropsidin A: A Pimarane Diterpene with Interesting Biological Activities and Promising Practical Applications.Chembiochem. 2021 Dec 2;22(23):3263-3269. doi: 10.1002/cbic.202100283. Epub 2021 Jul 30. Chembiochem. 2021. PMID: 34241944 Free PMC article. Review.
Cited by
-
Traversing the fungal terpenome.Nat Prod Rep. 2014 Oct;31(10):1449-73. doi: 10.1039/c4np00075g. Nat Prod Rep. 2014. PMID: 25171145 Free PMC article. Review.
-
Deciphering the chemical instability of sphaeropsidin A under physiological conditions - degradation studies and structural elucidation of the major metabolite.Org Biomol Chem. 2020 Oct 21;18(40):8147-8160. doi: 10.1039/d0ob01586e. Org Biomol Chem. 2020. PMID: 33016969 Free PMC article.
-
Putative Anticancer Compounds from Plant-Derived Endophytic Fungi: A Review.Molecules. 2022 Jan 4;27(1):296. doi: 10.3390/molecules27010296. Molecules. 2022. PMID: 35011527 Free PMC article. Review.
-
An unprecedented antioxidative isopimarane norditerpenoid from bivalve clam, Paphia malabarica with anti-cyclooxygenase and lipoxygenase potential.Pharm Biol. 2017 Dec;55(1):819-824. doi: 10.1080/13880209.2017.1280061. Pharm Biol. 2017. PMID: 28116944 Free PMC article.
-
Carotane sesquiterpenoids A-G from the desert endophytic fungus Fusarium sp. HM 166.RSC Adv. 2022 Aug 31;12(38):24590-24595. doi: 10.1039/d2ra02762c. eCollection 2022 Aug 30. RSC Adv. 2022. PMID: 36128376 Free PMC article.
References
-
-
Studies on Arid Lands Plants and Microorganisms, Part 22. For Part 21, see: Xu Y, Gao S, Bunting DP, Gunatilaka AAL. Phytochemistry. 2011;72:518–522.
-
-
- Turbyville TJ, Wijeratne EMK, Liu MX, Burns AM, Seliga CJ, Luevano LA, David CL, Feath SH, Whitesell L, Gunatilaka AAL. J Nat Prod. 2006;69:178–184. - PMC - PubMed
- Zhan J, Burns AM, Liu MX, Faeth SH, Gunatilaka AAL. J Nat Prod. 2007;70:227–232. - PMC - PubMed
- Paranagama PA, Wijeratne EMK, Burns AM, Marron MT, Gunatilaka MK, Arnold AE, Gunatilaka AAL. J Nat Prod. 2007;70:1700–1705. - PubMed
-
- Sporn MB. Lancet. 1996;347:1377–1381. - PubMed
-
- Christofori G. Nature. 2006;441:444–450. - PubMed
-
- Arnold AE. Fungal Biol Rev. 2007;21:51–66.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

