A malaria transmission-directed model of mosquito life cycle and ecology
- PMID: 21999664
- PMCID: PMC3224385
- DOI: 10.1186/1475-2875-10-303
A malaria transmission-directed model of mosquito life cycle and ecology
Abstract
Background: Malaria is a major public health issue in much of the world, and the mosquito vectors which drive transmission are key targets for interventions. Mathematical models for planning malaria eradication benefit from detailed representations of local mosquito populations, their natural dynamics and their response to campaign pressures.
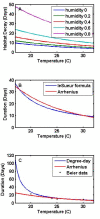
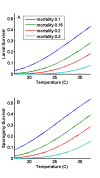
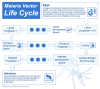
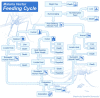
Methods: A new model is presented for mosquito population dynamics, effects of weather, and impacts of multiple simultaneous interventions. This model is then embedded in a large-scale individual-based simulation and results for local elimination of malaria are discussed. Mosquito population behaviours, such as anthropophily and indoor feeding, are included to study their effect upon the efficacy of vector control-based elimination campaigns.
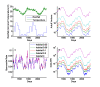
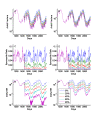
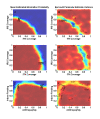
Results: Results for vector control tools, such as bed nets, indoor spraying, larval control and space spraying, both alone and in combination, are displayed for a single-location simulation with vector species and seasonality characteristic of central Tanzania, varying baseline transmission intensity and vector bionomics. The sensitivities to habitat type, anthropophily, indoor feeding, and baseline transmission intensity are explored.
Conclusions: The ability to model a spectrum of local vector species with different ecologies and behaviours allows local customization of packages of interventions and exploration of the effect of proposed new tools.
Figures

References
-
- Jacob BG, Muturi E, Halbig P, Mwangangi J, Wanjogu RK, Mpanga E, Funes J, Shililu J, Githure J, Regens JL, Novak RJ. Environmental abundance of Anopheles (Diptera: Culicidae) larval habitats on land cover change sites in Karima Village, Mwea Rice Scheme, Kenya. Am J Trop Med Hyg. 2007;76:73–80. - PubMed
-
- De Castro MC, Yamagata Y, Mtasiwa D, Tanner M, Utzinger J, Keiser J, Singer BH. Integrated urban malaria control: a case study in Dar Es Salaam, Tanzania. Am J Trop Med Hyg. 2004;71:103–117. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

