Domain loss has independently occurred multiple times in plant terpene synthase evolution
- PMID: 21999670
- PMCID: PMC3237789
- DOI: 10.1111/j.1365-313X.2011.04756.x
Domain loss has independently occurred multiple times in plant terpene synthase evolution
Abstract
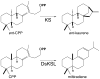
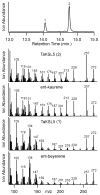
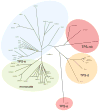
The extensive family of plant terpene synthases (TPSs) generally has a bi-domain structure, yet phylogenetic analyses consistently indicate that these synthases have evolved from larger diterpene synthases. In particular, that duplication of the diterpene synthase genes required for gibberellin phytohormone biosynthesis provided an early predecessor, whose loss of a approximately 220 amino acid 'internal sequence element' (now recognized as the γ domain) gave rise to the precursor of the modern mono- and sesqui-TPSs found in all higher plants. Intriguingly, TPSs are conserved by taxonomic relationships rather than function. This relationship demonstrates that such functional radiation has occurred both repeatedly and relatively recently, yet phylogenetic analyses assume that the 'internal/γ' domain loss represents a single evolutionary event. Here we provide evidence that such a loss was not a singular event, but rather has occurred multiple times. Specifically, we provide an example of a bi-domain diterpene synthase from Salvia miltiorrhiza, along with a sesquiterpene synthase from Triticum aestivum (wheat) that is not only closely related to diterpene synthases, but retains the ent-kaurene synthase activity relevant to the ancestral gibberellin metabolic function. Indeed, while the wheat sesquiterpene synthase clearly no longer contains the 'internal/γ' domain, it is closely related to rice diterpene synthase genes that retain the ancestral tri-domain structure. Thus, these findings provide examples of key evolutionary intermediates that underlie the bi-domain structure observed in the expansive plant TPS gene family, as well as indicating that 'internal/γ' domain loss has occurred independently multiple times, highlighting the complex evolutionary history of this important enzymatic family.
© 2011 The Authors. The Plant Journal © 2011 Blackwell Publishing Ltd.
Figures
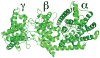




References
-
- Chen F, Tholl D, Bohlmann J, Pichersky E. The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011;66:212–229. - PubMed
-
- Cho EM, Okada A, Kenmoku H, Otomo K, Toyomasu T, Mitsuhashi W, Sassa T, Yajima A, Yabuta G, Mori K, Oikawa H, Toshima H, Shibuya N, Nojiri H, Omori T, Nishiyama M, Yamane H. Molecular cloning and characterization of a cDNA encoding ent-cassa-12,15-diene synthase, a putative diterpenoid phytoalexin biosynthetic enzyme, from suspension-cultured rice cells treated with a chitin elicitor. Plant J. 2004;37:1–8. - PubMed
-
- Christianson DW. Structural biology and chemistry of the terpenoid cyclases. Chem Rev. 2006;106:3412–3442. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

