Pioglitazone modulates vascular inflammation in atherosclerotic rabbits noninvasive assessment with FDG-PET-CT and dynamic contrast-enhanced MR imaging
- PMID: 21999870
- PMCID: PMC3253377
- DOI: 10.1016/j.jcmg.2011.04.020
Pioglitazone modulates vascular inflammation in atherosclerotic rabbits noninvasive assessment with FDG-PET-CT and dynamic contrast-enhanced MR imaging
Abstract
Objectives: We sought to determine the antiatherosclerotic properties of pioglitazone using multimethod noninvasive imaging techniques.
Background: Inflammation is an essential component of vulnerable or high-risk atheromas. Pioglitazone, a peroxisome proliferator-activated receptor-gamma agonist, possesses potent anti-inflammatory properties. We aimed to quantify noninvasively the anti-inflammatory effects of pioglitazone on atheroma using (18)F-fluorodeoxyglucose ((18)F-FDG) positron emission tomography (PET)/computed tomography (CT) and dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI).
Methods: Atherosclerotic plaques were induced in the aorta of 15 New Zealand white rabbits by a combination of a hyperlipidemic diet and 2 balloon endothelial denudations. Nine rabbits continued the same diet, whereas 6 rabbits received pioglitazone (10 mg/kg orally) in addition to the diet. Twelve animals underwent (18)F-FDG-PET/CT, and 15 animals underwent DCE-MRI at baseline, 1 month, and 3 months after treatment initiation. Concomitantly, serum metabolic parameters were monitored. After imaging was completed, aortic histologic analysis and correlation analysis were performed.
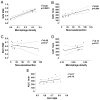
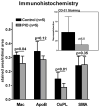
Results: The (18)F-FDG-PET/CT imaging detected an increase in average standardized uptake value in the control group (p < 0.01), indicating progressive inflammation, whereas stable standardized uptake values were observed in the treatment group, indicating no progression. The DCE-MRI analysis detected a significant decrease in the area under the curve for the pioglitazone group (p < 0.01). Immunohistologic examination of the aortas demonstrated a significant decrease in macrophage and oxidized phospholipid immunoreactivity in the pioglitazone group (p = 0.04 and p = 0.01, respectively) with respect to control animals, underlining the imaging results. Serum metabolic parameters showed no difference between groups. Strong positive correlations between standardized uptake value and macrophage density and between area under the curve and neovessels were detected (r(2) = 0.86 and p < 0.0001, and r(2) = 0.66 and p = 0.004, respectively).
Conclusions: Both (18)F-FDG-PET/CT and DCE-MRI demonstrate noninvasively the anti-inflammatory effects of pioglitazone on atheroma. Both imaging methods seem suited to monitor inflammation in atherosclerosis.
Copyright © 2011 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures





Comment in
-
Imaging inflammatory changes in atherosclerosis multimodal imaging hitting stride.JACC Cardiovasc Imaging. 2011 Oct;4(10):1119-22. doi: 10.1016/j.jcmg.2011.09.001. JACC Cardiovasc Imaging. 2011. PMID: 21999872 No abstract available.
References
-
- Dahlöf B. Cardiovascular disease risk factors: Epidemiology and risk assessment. Amer J Cardiol. 2010;105:3A–9A. - PubMed
-
- Ross R. Atherosclerosis -- An Inflammatory Disease. N Engl J Med. 1999;340:115–126. - PubMed
-
- Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and High-Risk Plaque: Part I: Evolving Concepts. J Am Col Cardiol. 2005;46:937–954. - PubMed
-
- Natarajan P, Ray KK, Cannon CP. High-Density Lipoprotein and coronary heart disease: Current and future therapies. J Am Coll of Cardiol. 2010;55:1283–1299. - PubMed

