A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA
- PMID: 22000014
- PMCID: PMC3234495
- DOI: 10.1016/j.cell.2011.09.028
A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA
Erratum in
- Cell. 2011 Nov 11;147(4):947
Abstract
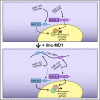
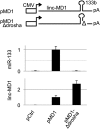
Recently, a new regulatory circuitry has been identified in which RNAs can crosstalk with each other by competing for shared microRNAs. Such competing endogenous RNAs (ceRNAs) regulate the distribution of miRNA molecules on their targets and thereby impose an additional level of post-transcriptional regulation. Here we identify a muscle-specific long noncoding RNA, linc-MD1, which governs the time of muscle differentiation by acting as a ceRNA in mouse and human myoblasts. Downregulation or overexpression of linc-MD1 correlate with retardation or anticipation of the muscle differentiation program, respectively. We show that linc-MD1 "sponges" miR-133 and miR-133 [corrected] to regulate the expression of MAML1 and MEF2C, transcription factors that activate muscle-specific gene expression. Finally, we demonstrate that linc-MD1 exerts the same control over differentiation timing in human myoblasts, and that its levels are strongly reduced in Duchenne muscle cells. We conclude that the ceRNA network plays an important role in muscle differentiation.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures













Comment in
-
RNA: a new layer of regulation.Nat Rev Mol Cell Biol. 2011 Nov 3;12(12):766. doi: 10.1038/nrm3225. Nat Rev Mol Cell Biol. 2011. PMID: 22048709 No abstract available.
-
ceRNAs: miRNA target mimic mimics.Cell. 2011 Dec 23;147(7):1431-2. doi: 10.1016/j.cell.2011.12.003. Cell. 2011. PMID: 22196719 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

