Systematic discovery of Rab GTPases with synaptic functions in Drosophila
- PMID: 22000105
- PMCID: PMC3351199
- DOI: 10.1016/j.cub.2011.08.058
Systematic discovery of Rab GTPases with synaptic functions in Drosophila
Abstract
Background: Neurons require highly specialized intracellular membrane trafficking, especially at synapses. Rab GTPases are considered master regulators of membrane trafficking in all cells, and only very few Rabs have known neuron-specific functions. Here, we present the first systematic characterization of neuronal expression, subcellular localization, and function of Rab GTPases in an organism with a brain.
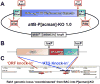
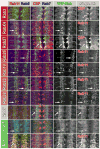
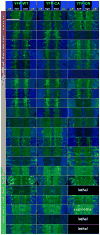
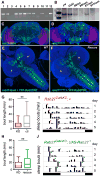
Results: We report the surprising discovery that half of all Drosophila Rabs function specifically or predominantly in distinct subsets of neurons in the brain. Furthermore, functional profiling of the GTP/GDP-bound states reveals that these neuronal Rabs are almost exclusively active at synapses and the majority of these synaptic Rabs specifically mark synaptic recycling endosomal compartments. Our profiling strategy is based on Gal4 knockins in large genomic fragments that are additionally designed to generate mutants by ends-out homologous recombination. We generated 36 large genomic targeting vectors and transgenic rab-Gal4 fly strains for 25 rab genes. Proof-of-principle knockout of the synaptic rab27 reveals a sleep phenotype that matches its cell-specific expression.
Conclusions: Our findings suggest that up to half of all Drosophila Rabs exert specialized synaptic functions. The tools presented here allow systematic functional studies of these Rabs and provide a method that is applicable to any large gene family in Drosophila.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Vesicle trafficking: a Rab family profile.Curr Biol. 2011 Oct 25;21(20):R841-3. doi: 10.1016/j.cub.2011.08.061. Curr Biol. 2011. PMID: 22032185
Similar articles
-
Vesicle trafficking: a Rab family profile.Curr Biol. 2011 Oct 25;21(20):R841-3. doi: 10.1016/j.cub.2011.08.061. Curr Biol. 2011. PMID: 22032185
-
Systematic functional analysis of rab GTPases reveals limits of neuronal robustness to environmental challenges in flies.Elife. 2021 Mar 5;10:e59594. doi: 10.7554/eLife.59594. Elife. 2021. PMID: 33666175 Free PMC article.
-
Comprehensive functional analysis of Rab GTPases in Drosophila nephrocytes.Cell Tissue Res. 2017 Jun;368(3):615-627. doi: 10.1007/s00441-017-2575-2. Epub 2017 Feb 8. Cell Tissue Res. 2017. PMID: 28180992 Free PMC article.
-
Rab family of small GTPases: an updated view on their regulation and functions.FEBS J. 2021 Jan;288(1):36-55. doi: 10.1111/febs.15453. Epub 2020 Jul 1. FEBS J. 2021. PMID: 32542850 Free PMC article. Review.
-
Rab GTPases and their roles in brain neurons and glia.Brain Res Rev. 2008 Jun;58(1):236-46. doi: 10.1016/j.brainresrev.2008.04.006. Epub 2008 Apr 12. Brain Res Rev. 2008. PMID: 18485483 Review.
Cited by
-
Maintenance of glia in the optic lamina is mediated by EGFR signaling by photoreceptors in adult Drosophila.PLoS Genet. 2015 Apr 24;11(4):e1005187. doi: 10.1371/journal.pgen.1005187. eCollection 2015 Apr. PLoS Genet. 2015. PMID: 25909451 Free PMC article.
-
The GTPase Rab26 links synaptic vesicles to the autophagy pathway.Elife. 2015 Feb 2;4:e05597. doi: 10.7554/eLife.05597. Elife. 2015. PMID: 25643395 Free PMC article.
-
Gene Tagging Strategies To Assess Protein Expression, Localization, and Function in Drosophila.Genetics. 2017 Oct;207(2):389-412. doi: 10.1534/genetics.117.199968. Genetics. 2017. PMID: 28978772 Free PMC article. Review.
-
Lifespan regulation in α/β posterior neurons of the fly mushroom bodies by Rab27.Aging Cell. 2020 Aug;19(8):e13179. doi: 10.1111/acel.13179. Epub 2020 Jul 6. Aging Cell. 2020. PMID: 32627932 Free PMC article.
-
Recombineering homologous recombination constructs in Drosophila.J Vis Exp. 2013 Jul 13;(77):e50346. doi: 10.3791/50346. J Vis Exp. 2013. PMID: 23893070 Free PMC article.
References
-
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. - PubMed
-
- Pfeffer SR. Unsolved mysteries in membrane traffic. Annu Rev Biochem. 2007;76:629–645. - PubMed
-
- Pfeffer SR. Rab GTPases: master regulators of membrane trafficking. Curr Opin Cell Biol. 1994;6:522–526. - PubMed
-
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

