Advances in structural and functional analysis of membrane proteins by electron crystallography
- PMID: 22000511
- PMCID: PMC3197218
- DOI: 10.1016/j.str.2011.09.001
Advances in structural and functional analysis of membrane proteins by electron crystallography
Abstract
Electron crystallography is a powerful technique for the study of membrane protein structure and function in the lipid environment. When well-ordered two-dimensional crystals are obtained the structure of both protein and lipid can be determined and lipid-protein interactions analyzed. Protons and ionic charges can be visualized by electron crystallography and the protein of interest can be captured for structural analysis in a variety of physiologically distinct states. This review highlights the strengths of electron crystallography and the momentum that is building up in automation and the development of high throughput tools and methods for structural and functional analysis of membrane proteins by electron crystallography.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures




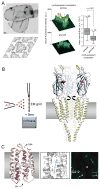

References
-
- Appel M, Hizlan D, Vinothkumar KR, Ziegler C, Kuhlbrandt W. Conformations of NhaA, the Na/H exchanger from Escherichia coli, in the pH-activated and ion-translocating states. Journal of molecular biology. 2009;386:351–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

