Acetaminophen-induced hepatotoxicity and protein nitration in neuronal nitric-oxide synthase knockout mice
- PMID: 22001257
- PMCID: PMC3251022
- DOI: 10.1124/jpet.111.184192
Acetaminophen-induced hepatotoxicity and protein nitration in neuronal nitric-oxide synthase knockout mice
Abstract
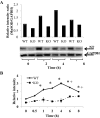
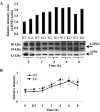
In overdose acetaminophen (APAP) is hepatotoxic. Toxicity occurs by metabolism to N-acetyl-p-benzoquinone imine, which depletes GSH and covalently binds to proteins followed by protein nitration. Nitration can occur via the strong oxidant and nitrating agent peroxynitrite, formed from superoxide and nitric oxide (NO). In hepatocyte suspensions we reported that an inhibitor of neuronal nitric-oxide synthase (nNOS; NOS1), which has been reported to be in mitochondria, inhibited toxicity and protein nitration. We recently showed that manganese superoxide dismutase (MnSOD; SOD2) was nitrated and inactivated in APAP-treated mice. To understand the role of nNOS in APAP toxicity and MnSOD nitration, nNOS knockout (KO) and wild-type (WT) mice were administered APAP (300 mg/kg). In WT mice serum alanine aminotransferase (ALT) significantly increased at 6 and 8 h, and serum aspartate aminotransferase (AST) significantly increased at 4, 6 and 8 h; however, in KO mice neither ALT nor AST significantly increased until 8 h. There were no significant differences in hepatic GSH depletion, APAP protein binding, hydroxynonenal covalent binding, or histopathological assessment of toxicity. The activity of hepatic MnSOD was significantly lower at 1 to 2 h in WT mice and subsequently increased at 8 h. MnSOD activity was not altered at 0 to 6 h in KO mice but was significantly decreased at 8 h. There were significant increases in MnSOD nitration at 1 to 8 h in WT mice and 6 to 8 h in KO mice. Significantly more nitration occurred at 1 to 6 h in WT than in KO mice. MnSOD was the only observed nitrated protein after APAP treatment. These data indicate a role for nNOS with inactivation of MnSOD and ALT release during APAP toxicity.
Figures





References
-
- Amimoto T, Matsura T, Koyama SY, Nakanishi T, Yamada K, Kajiyama G. (1995) Acetaminophen-induced hepatic injury in mice: the role of lipid peroxidation and effects of pretreatment with coenzyme Q10 and α-tocopherol. Free Radic Biol Med 19:169–176 - PubMed
-
- Boobis AR, Seddon CE, Nasseri-Sina P, Davies DS. (1990) Evidence for a direct role of intracellular calcium in paracetamol toxicity. Biochem Pharmacol 39:1277–1281 - PubMed
-
- Boobis AR, Tee LB, Hampden CE, Davies DS. (1986) Freshly isolated hepatocytes as a model for studying the toxicity of paracetamol. Food Chem Toxicol 24:731–736 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

