Strategies for optimizing the serum persistence of engineered human arginase I for cancer therapy
- PMID: 22001609
- PMCID: PMC3294146
- DOI: 10.1016/j.jconrel.2011.09.097
Strategies for optimizing the serum persistence of engineered human arginase I for cancer therapy
Abstract
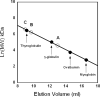
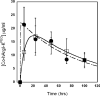
Systemic L-arginine depletion following intravenous administration of l-arginine hydrolyzing enzymes has been shown to selectively impact tumors displaying urea cycle defects including a large fraction of hepatocellular carcinomas, metastatic melanomas and small cell lung carcinomas. However, the human arginases display poor serum stability (t(1/2)=4.8h) whereas a bacterial arginine deiminase evaluated in phase II clinical trials was reported to be immunogenic, eliciting strong neutralizing antibody responses. Recently, we showed that substitution of the Mn(2+) metal center in human Arginase I with Co(2+) (Co-hArgI) results in an enzyme that displays 10-fold higher catalytic efficiency for L-Arg hydrolysis, 12-15 fold reduction in the IC(50) towards a variety of malignant cell lines and, importantly a t(1/2)=22h in serum. To investigate the utility of Co-hArgI for L-Arg depletion therapy in cancer we systematically investigated three strategies for enhancing the persistence of the enzyme in circulation: (i) site specific conjugation of Co-hArgI engineered with an accessible N-terminal Cys residue to 20kDa PEG-maleimide (Co-hArgI-C(PEG-20K)); (ii) engineering of the homotrimeric Co-hArgI into a linked, monomeric 110kDa polypeptide (Co-hArgI x3) and (iii) lysyl conjugation of 5kDa PEG-N-hydroxysuccinimide (NHS) ester (Co-hArgI-K(PEG-5K)). Surprisingly, even though all three formulations resulted in proteins with a predicted hydrodynamic radius larger than the cut-off for renal filtration, only Co-hArgI amine conjugated to 5kDa PEG remained in circulation for sufficiently long durations. Using Co-hArgI-K(PEG-5K) labeled with an end-terminal fluorescein for easy detection, we demonstrated that following intraperitoneal administration at 6mg/kg weight, a well tolerated dose, the circulation t(1/2) of the protein in Balb/c mice is 63±10h. Very low levels of serum L-Arg (<5μM) could be sustained for over 75h after injection, representing a 9-fold increase in pharmacodynamic efficacy relative to similarly prepared Mn(2+)-containing hArgI conjugated to 5kDa PEG-NHS ester (Mn-hArgI-K(PEG-5K)). The favorable pharmacokinetic and pharmacodynamic properties of Co-hArgI-K(PEG-5K) reported here, coupled with its human origin which should reduce the likelihood of adverse immune responses, make it a promising candidate for cancer therapy.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures

References
-
- Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002;62(19):5443–5450. - PubMed
-
- Yoon CY, Shim YJ, Kim EH, Lee JH, Won NH, Kim JH, Park IS, Yoon DK, Min BH. Renal cell carcinoma does not express argininosuccinate synthetase and is highly sensitive to arginine deprivation via arginine deiminase. Int J Cancer. 2007;120(4):897–905. - PubMed
-
- Cheng N, Chytil A, Shyr Y, Joly A, Moses HL. Enhanced hepatocyte growth factor signaling by type II transforming growth factor-beta receptor knockout fibroblasts promotes mammary tumorigenesis. Cancer Res. 2007;67(10):4869–4877. - PubMed
-
- Shen LJ, Beloussow K, Shen WC. Modulation of arginine metabolic pathways as the potential anti-tumor mechanism of recombinant arginine deiminase. Cancer Lett. 2006;231(1):30–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

