Expression of soluble and functional full-length human matrix metalloproteinase-2 in Escherichia coli
- PMID: 22001844
- PMCID: PMC3462441
- DOI: 10.1016/j.jbiotec.2011.09.030
Expression of soluble and functional full-length human matrix metalloproteinase-2 in Escherichia coli
Abstract
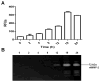
Characterization of the matrix metalloproteinase-2 (MMP-2) substrates and understanding of its function remain difficult because up to date preparations containing minor amounts of other eukaryotic proteins that are co-purified with MMP-2 are still used. In this work, the expression of a soluble and functional full-length recombinant human MMP-2 (rhMMP-2) in the cytoplasm of Escherichia coli is reported, and the purification of this metalloproteinase is described. Culture of this bacterium at 18°C culminated in maintenance of the soluble and functional rhMMP-2 in the soluble fraction of the E. coli lysate and its purification by affinity with gelatin-sepharose yielded approximately 0.12mg/L of medium. Western Blotting and zymographic analysis revealed that the most abundant form was the 72-kDa MMP-2, but some gelatinolytic bands corresponding to proteins with lower molecular weight were also detected. The obtained rhMMP-2 was demonstrated to be functional in a gelatinolytic fluorimetric assay, suggesting that the purified rhMMP-2 was correctly folded. The method described here involves fewer steps, is less expensive, and is less prone to contamination with other proteinases and MMP inhibitors as compared to expression of rhMMP-2 in eukaryotic tissue culture. This protocol will facilitate the use of the full-length rhMMP-2 expressed in bacteria and will certainly help researchers to acquire new knowledge about the substrates and biological activities of this important proteinase.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures

References
-
- Back M, Ketelhuth DF, Agewall S. Matrix metalloproteinases in atherothrombosis. Prog Cardiovasc Dis. 2010;52:410–428. - PubMed
-
- Baneyx F, Mujacic M. Recombinant protein folding and misfolding in Escherichia coli. Nat Biotechnol. 2004;22:1399–1408. - PubMed
-
- Burgess RR. Refolding solubilized inclusion body proteins. Methods Enzymol. 2009;463:259–282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

