Fat-induced membrane cholesterol accrual provokes cortical filamentous actin destabilisation and glucose transport dysfunction in skeletal muscle
- PMID: 22002007
- PMCID: PMC3245823
- DOI: 10.1007/s00125-011-2334-y
Fat-induced membrane cholesterol accrual provokes cortical filamentous actin destabilisation and glucose transport dysfunction in skeletal muscle
Abstract
Aims/hypothesis: Diminished cortical filamentous actin (F-actin) has been implicated in skeletal muscle insulin resistance, yet the mechanism(s) is unknown. Here we tested the hypothesis that changes in membrane cholesterol could be a causative factor, as organised F-actin structure emanates from cholesterol-enriched raft microdomains at the plasma membrane.
Methods: Skeletal muscle samples from high-fat-fed animals and insulin-sensitive and insulin-resistant human participants were evaluated. The study also used L6 myotubes to directly determine the impact of fatty acids (FAs) on membrane/cytoskeletal variables and insulin action.
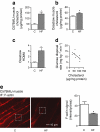
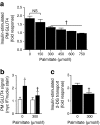
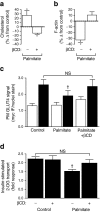
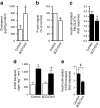
Results: High-fat-fed insulin-resistant animals displayed elevated levels of membrane cholesterol and reduced F-actin structure compared with normal chow-fed animals. Moreover, human muscle biopsies revealed an inverse correlation between membrane cholesterol and whole-body glucose disposal. Palmitate-induced insulin-resistant myotubes displayed membrane cholesterol accrual and F-actin loss. Cholesterol lowering protected against the palmitate-induced defects, whereas characteristically measured defects in insulin signalling were not corrected. Conversely, cholesterol loading of L6 myotube membranes provoked a palmitate-like cytoskeletal/GLUT4 derangement. Mechanistically, we observed a palmitate-induced increase in O-linked glycosylation, an end-product of the hexosamine biosynthesis pathway (HBP). Consistent with HBP activity affecting the transcription of various genes, we observed an increase in Hmgcr, a gene that encodes 3-hydroxy-3-methyl-glutaryl coenzyme A reductase, the rate-limiting enzyme in cholesterol synthesis. In line with increased HBP activity transcriptionally provoking a membrane cholesterol-based insulin-resistant state, HBP inhibition attenuated Hmgcr expression and prevented membrane cholesterol accrual, F-actin loss and GLUT4/glucose transport dysfunction.
Conclusions/interpretation: Our results suggest a novel cholesterolgenic-based mechanism of FA-induced membrane/cytoskeletal disorder and insulin resistance.
Figures

Similar articles
-
Evidence coupling increased hexosamine biosynthesis pathway activity to membrane cholesterol toxicity and cortical filamentous actin derangement contributing to cellular insulin resistance.Endocrinology. 2011 Sep;152(9):3373-84. doi: 10.1210/en.2011-1295. Epub 2011 Jun 28. Endocrinology. 2011. PMID: 21712361 Free PMC article.
-
An early, reversible cholesterolgenic etiology of diet-induced insulin resistance.Mol Metab. 2023 Jun;72:101715. doi: 10.1016/j.molmet.2023.101715. Epub 2023 Apr 3. Mol Metab. 2023. PMID: 37019209 Free PMC article.
-
Chromium enhances insulin responsiveness via AMPK.J Nutr Biochem. 2014 May;25(5):565-72. doi: 10.1016/j.jnutbio.2014.01.007. Epub 2014 Feb 20. J Nutr Biochem. 2014. PMID: 24725432 Free PMC article.
-
Membrane Cholesterol in Skeletal Muscle: A Novel Player in Excitation-Contraction Coupling and Insulin Resistance.J Diabetes Res. 2017;2017:3941898. doi: 10.1155/2017/3941898. Epub 2017 Mar 6. J Diabetes Res. 2017. PMID: 28367451 Free PMC article. Review.
-
"Actin"g on GLUT4: membrane & cytoskeletal components of insulin action.Curr Diabetes Rev. 2007 May;3(2):111-22. doi: 10.2174/157339907780598199. Curr Diabetes Rev. 2007. PMID: 18220662 Free PMC article. Review.
Cited by
-
A nexus of lipid and O-Glcnac metabolism in physiology and disease.Front Endocrinol (Lausanne). 2022 Aug 30;13:943576. doi: 10.3389/fendo.2022.943576. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36111295 Free PMC article. Review.
-
Tropomodulin3 is a novel Akt2 effector regulating insulin-stimulated GLUT4 exocytosis through cortical actin remodeling.Nat Commun. 2015 Jan 9;6:5951. doi: 10.1038/ncomms6951. Nat Commun. 2015. PMID: 25575350 Free PMC article.
-
AMPK enhances insulin-stimulated GLUT4 regulation via lowering membrane cholesterol.Endocrinology. 2012 May;153(5):2130-41. doi: 10.1210/en.2011-2099. Epub 2012 Mar 20. Endocrinology. 2012. PMID: 22434076 Free PMC article.
-
The Nutrient-Sensing Hexosamine Biosynthetic Pathway as the Hub of Cancer Metabolic Rewiring.Cells. 2018 Jun 2;7(6):53. doi: 10.3390/cells7060053. Cells. 2018. PMID: 29865240 Free PMC article. Review.
-
Reconstructing the blood metabolome and genotype using long-range chromatin interactions.Metabol Open. 2020 Mar 19;6:100035. doi: 10.1016/j.metop.2020.100035. eCollection 2020 Jun. Metabol Open. 2020. PMID: 32812909 Free PMC article.
References
-
- Perseghin G, Scifo P, De Cobelli F, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. - DOI - PubMed
-
- Rizza RA, Mandarino LJ, Genest J, Baker BA, Gerich JE. Production of insulin resistance by hyperinsulinaemia in man. Diabetologia. 1985;28:70–75. - PubMed
-
- McGarry JD. Glucose-fatty acid interactions in health and disease. Am J Clin Nutr. 1998;67:500S–504S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

