Metabolism and accumulation of the lipophilic deoxynucleoside analogs elacytarabine and CP-4126
- PMID: 22002019
- PMCID: PMC3432794
- DOI: 10.1007/s10637-011-9756-8
Metabolism and accumulation of the lipophilic deoxynucleoside analogs elacytarabine and CP-4126
Abstract
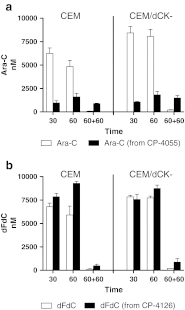
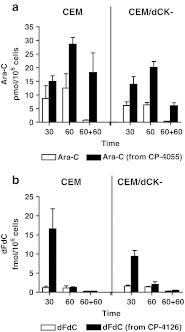
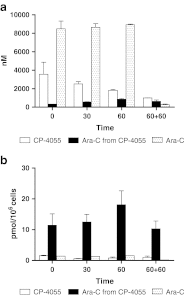
Cytarabine (ara-C) and gemcitabine (dFdC) are commonly used anticancer drugs, which depend on the equilibrative (ENT) and concentrative-nucleoside-transporters to enter the cell. To bypass transport-related drug resistance, lipophilic derivatives elacytarabine (CP-4055), ara-C-5'elaidic-acid-ester, and CP-4126, (CO 1.01) gemcitabine-5'elaidic-acid-ester, were investigated for the entry into the cell, distribution, metabolism and retention. The leukemic CEM-cell-line and its deoxycytidine-kinase deficient variant (CEM/dCK-) were exposed for 30 and 60 min to the radiolabeled drugs; followed by culture in drug-free medium in order to determine drug retention in the cell. The cellular fractions were analyzed with thin-layer-chromatography and HPLC. Elacytarabine and CP-4126 were converted to the parent compounds both inside and outside the cell (35-45%). The ENT-inhibitor dipyridamole did not affect their uptake or retention. Inside the cell Elacytarabine and CP-4126 predominantly localized in the membrane and cytosolic fraction, leading to a long retention after removal of the medium. In contrast, in cells exposed to the parent drugs ara-C and dFdC, intracellular drug concentration increased during exposure but decreased to undetectable levels after drug removal. In the dCK- cell line, no metabolism was observed. The concentrations of ara-CTP and dFdCTP reached a peak at the end of the incubation with the drugs, and decreased after drug removal; peak levels of dFdCTP were 35 times higher than ara-CTP and was retained better. In contrast, after exposure to elacytarabine or CP-4126, ara-CTP and dFdCTP levels continued to increase not only during exposure but also during 120 min after removal of the elacytarabine and CP-4126. Levels of ara-CTP and dFdCTP were higher than after exposure to the parent drugs. In conclusion, the lipophilic derivatives elacytarabine and CP-4126 showed a nucleoside-transporter independent uptake, with long retention of the active nucleotides. These lipophilic nucleoside analogues are new chemical entities suitable for novel clinical applications.
Figures

Similar articles
-
Induction of resistance to the lipophilic cytarabine prodrug elacytarabine (CP-4055) in CEM leukemic cells.Nucleosides Nucleotides Nucleic Acids. 2010 Jun;29(4-6):394-9. doi: 10.1080/15257771003741166. Nucleosides Nucleotides Nucleic Acids. 2010. PMID: 20544525
-
Mechanisms of uptake and resistance to troxacitabine, a novel deoxycytidine nucleoside analogue, in human leukemic and solid tumor cell lines.Cancer Res. 2001 Oct 1;61(19):7217-24. Cancer Res. 2001. PMID: 11585758
-
The activity of the lipophilic nucleoside derivatives elacytarabine and CP-4126 in a panel of tumor cell lines resistant to nucleoside analogues.Nucleosides Nucleotides Nucleic Acids. 2010 Jun;29(4-6):386-93. doi: 10.1080/15257771003729625. Nucleosides Nucleotides Nucleic Acids. 2010. PMID: 20544524
-
Elacytarabine--lipid vector technology overcoming drug resistance in acute myeloid leukemia.Expert Opin Investig Drugs. 2011 Dec;20(12):1707-15. doi: 10.1517/13543784.2011.625009. Epub 2011 Nov 1. Expert Opin Investig Drugs. 2011. PMID: 22040175 Review.
-
Clinical potential of elacytarabine in patients with acute myeloid leukemia.Ther Adv Hematol. 2014 Dec;5(6):211-20. doi: 10.1177/2040620714552615. Ther Adv Hematol. 2014. PMID: 25469211 Free PMC article. Review.
Cited by
-
Advance of structural modification of nucleosides scaffold.Eur J Med Chem. 2021 Mar 15;214:113233. doi: 10.1016/j.ejmech.2021.113233. Epub 2021 Jan 30. Eur J Med Chem. 2021. PMID: 33550179 Free PMC article. Review.
-
Intestinal OCTN2- and MCT1-targeted drug delivery to improve oral bioavailability.Asian J Pharm Sci. 2020 Mar;15(2):158-173. doi: 10.1016/j.ajps.2020.02.002. Epub 2020 Mar 4. Asian J Pharm Sci. 2020. PMID: 32256846 Free PMC article. Review.
-
Elacytarabine has single-agent activity in patients with advanced acute myeloid leukaemia.Br J Haematol. 2012 Sep;158(5):581-8. doi: 10.1111/j.1365-2141.2012.09186.x. Epub 2012 Jun 15. Br J Haematol. 2012. PMID: 22702906 Free PMC article. Clinical Trial.
-
Antiproliferative and apoptotic activity of gemcitabine-lauric acid conjugate on human bladder cancer cells.Iran J Basic Med Sci. 2022 Apr;25(4):536-542. doi: 10.22038/IJBMS.2022.61118.13528. Iran J Basic Med Sci. 2022. PMID: 35656081 Free PMC article.
-
Improving nucleoside analogs via lipid conjugation: Is fatter any better?Crit Rev Oncol Hematol. 2016 Apr;100:46-56. doi: 10.1016/j.critrevonc.2016.01.015. Epub 2016 Jan 21. Crit Rev Oncol Hematol. 2016. PMID: 26829896 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

