Complex regulation of p73 isoforms after alteration of amyloid precursor polypeptide (APP) function and DNA damage in neurons
- PMID: 22002055
- PMCID: PMC3234838
- DOI: 10.1074/jbc.M111.261271
Complex regulation of p73 isoforms after alteration of amyloid precursor polypeptide (APP) function and DNA damage in neurons
Abstract
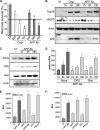
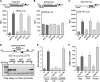
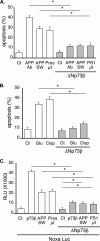
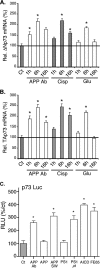
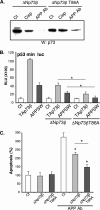
Genetic ablations of p73 have shown its implication in the development of the nervous system. However, the relative contribution of ΔNp73 and TAp73 isoforms in neuronal functions is still unclear. In this study, we have analyzed the expression of these isoforms during neuronal death induced by alteration of the amyloid-β precursor protein function or cisplatin. We observed a concomitant up-regulation of a TAp73 isoform and a down-regulation of a ΔNp73 isoform. The shift in favor of the pro-apoptotic isoform correlated with an induction of the p53/p73 target genes such as Noxa. At a functional level, we showed that TAp73 induced neuronal death and that ΔNp73 has a neuroprotective role toward amyloid-β precursor protein alteration or cisplatin. We investigated the mechanisms of p73 expression and found that the TAp73 expression was regulated at the promoter level. In contrast, regulation of ΔNp73 protein levels was regulated by phosphorylation at residue 86 and multiple proteases. Thus, this study indicates that tight transcriptional and post-translational mechanisms regulate the p73 isoform ratios that play an important role in neuronal survival.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

