Regulation of fertilization in male rats by CatSper2 knockdown
- PMID: 22002435
- PMCID: PMC3735093
- DOI: 10.1038/aja.2011.118
Regulation of fertilization in male rats by CatSper2 knockdown
Abstract
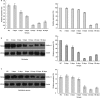
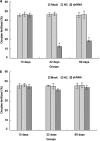
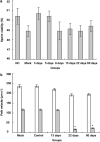
Interest in ion channels as drug targets for contraception has grown with the realization that certain ion channel subunits are located exclusively in sperm. Selective knockdown of ion channel subunits can lead to infertility without ill effects, and selective inhibitors and/or openers of these ion channels could interfere with sperm function. In this study, in vivo electroporation (EP) and rete testis microinjection-mediated plasmid DNA were adopted to silence CatSper2 expression, which is essential in sperm hyperactivation. The results showed that high transfection efficiency and expression were achieved by plasmid DNA that was directly injected into the rete testis. As a result of the expression of CatSper2 being blocked, the treatment group showed significantly lower (P<0.05) hyperactivation rate, fertilization rate in vitro, migration motility in viscoelastic solution and intracellular Ca(2+) peak. The low hyperactivation and fertilization rates lasted for 60 days. Meanwhile, analysis of the sperm survival rate and testis histology indicated that in vivo EP had no significant effect on the function of the testis, spermatogenesis or sperm activity. The present study demonstrated that it was feasible to achieve male contraception by silencing the expression of CatSper2, the key protein involved in sperm hyperactivation.
Figures


Similar articles
-
Identical phenotypes of CatSper1 and CatSper2 null sperm.J Biol Chem. 2005 Sep 16;280(37):32238-44. doi: 10.1074/jbc.M501430200. Epub 2005 Jul 21. J Biol Chem. 2005. PMID: 16036917
-
Hyperactivated sperm motility driven by CatSper2 is required for fertilization.Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):14869-74. doi: 10.1073/pnas.2136654100. Epub 2003 Dec 1. Proc Natl Acad Sci U S A. 2003. PMID: 14657366 Free PMC article.
-
A novel copy number variation in CATSPER2 causes idiopathic male infertility with normal semen parameters.Hum Reprod. 2019 Mar 1;34(3):414-423. doi: 10.1093/humrep/dey377. Hum Reprod. 2019. PMID: 30629171
-
CatSper channel, sperm function and male fertility.Reprod Biomed Online. 2015 Jan;30(1):28-38. doi: 10.1016/j.rbmo.2014.09.014. Epub 2014 Oct 8. Reprod Biomed Online. 2015. PMID: 25457194 Review.
-
pH Homeodynamics and Male Fertility: A Coordinated Regulation of Acid-Based Balance during Sperm Journey to Fertilization.Biomolecules. 2024 Jun 12;14(6):685. doi: 10.3390/biom14060685. Biomolecules. 2024. PMID: 38927088 Free PMC article. Review.
Cited by
-
Roles of CatSper channels in the pathogenesis of asthenozoospermia and the therapeutic effects of acupuncture-like treatment on asthenozoospermia.Theranostics. 2021 Jan 1;11(6):2822-2844. doi: 10.7150/thno.51869. eCollection 2021. Theranostics. 2021. PMID: 33456575 Free PMC article.
-
Testis-specific Fank1 gene in knockdown mice produces oligospermia via apoptosis.Asian J Androl. 2014 Jan-Feb;16(1):124-30. doi: 10.4103/1008-682X.122592. Asian J Androl. 2014. PMID: 24369145 Free PMC article.
-
Loss-of-function mutations in CEP78 cause male infertility in humans and mice.Sci Adv. 2022 Oct 7;8(40):eabn0968. doi: 10.1126/sciadv.abn0968. Epub 2022 Oct 7. Sci Adv. 2022. PMID: 36206347 Free PMC article.
References
-
- Anderson RA, Baird DT. Male contraception. Endocr Rev. 2002;23:735–62. - PubMed
-
- Kamischke A, Nieschlag E. Progress towards hormonal male contraception. Trends Pharmacol Sci. 2004;25:49–57. - PubMed
-
- WHO Task Force on Methods for the Regulation of Male Fertility Contraceptive efficacy of testosterone-induced azoospermia in normal men. Lancet. 1990;336:955–9. - PubMed
-
- Lopez LM, Grimes DA, Schulz KF. Nonhormonal drugs for contraception in men: a systematic review. Obstetr Gynecol Surv. 2005;60:746–52. - PubMed
-
- Delves PJ, Lund T, Roitt IM. Antifertility vaccines. Trends Immunol. 2002;23:213–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

