Brief treatment with iNKT cell ligand α-galactosylceramide confers a long-term protection against lupus
- PMID: 22002593
- PMCID: PMC3432318
- DOI: 10.1007/s10875-011-9590-y
Brief treatment with iNKT cell ligand α-galactosylceramide confers a long-term protection against lupus
Abstract
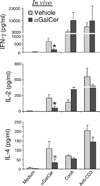
CD1d presents glycolipid antigens such as α-galactosylceramide (αGalCer) to invariant natural killer T cells (iNKT). We have reported that activated iNKTs inhibit IL-10-producing autoreactive B cells, while promoting or leaving intact the normal B cell responses, making iNKT modulation an attractive therapeutic modality. Here, we report that a brief treatment of young lupus-prone (NZB/NZW)F1 (BWF1) mice with two injections of αGalCer conferred a long-term protection against lupus. Long-term repeated administrations of αGalCer, however, afforded no clinical benefit. These disparate clinical effects correlated with iNKT responsiveness. While a brief treatment with αGalCer enhanced iNKT responses upon in vitro recall, the long-term αGalCer treatment resulted in reduced iNKT responses in BWF1 mice. The improvement in disease with αGalCer treatment was associated with the reduced IL-10 production. Furthermore, iNKTs directly inhibited IL-10-secreting cells in vivo in reconstituted SCID mice and inhibited IL-10-secreting B cells in vitro in co-cultures. Thus, a brief treatment with a CD1d-binding glycolipid enhances iNKT responses, reduces IL-10 production, and delays the onset of lupus, whereas long-term repeated treatments induce marked iNKT hyporesponsiveness and do not affect disease outcome in BWF1 mice. Identifying glycolipid regimens that can modulate iNKT responsiveness will have important implications for developing iNKT-based therapies for autoimmune diseases.
Figures





Similar articles
-
Examining the role of CD1d and natural killer T cells in the development of nephritis in a genetically susceptible lupus model.Arthritis Rheum. 2007 Apr;56(4):1219-33. doi: 10.1002/art.22490. Arthritis Rheum. 2007. PMID: 17393451 Free PMC article.
-
Invariant NKT cells inhibit autoreactive B cells in a contact- and CD1d-dependent manner.J Immunol. 2011 Feb 1;186(3):1512-20. doi: 10.4049/jimmunol.1002373. Epub 2011 Jan 5. J Immunol. 2011. PMID: 21209282 Free PMC article.
-
PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells.J Immunol. 2009 Mar 1;182(5):2816-26. doi: 10.4049/jimmunol.0803648. J Immunol. 2009. PMID: 19234176 Free PMC article.
-
The role of iNKT cells in the immunopathology of systemic lupus erythematosus.Ann N Y Acad Sci. 2009 Sep;1173:435-41. doi: 10.1111/j.1749-6632.2009.04743.x. Ann N Y Acad Sci. 2009. PMID: 19758183 Review.
-
Role of invariant natural killer T (iNKT) cells in systemic lupus erythematosus.Curr Med Chem. 2008;15(18):1778-87. doi: 10.2174/092986708785132988. Curr Med Chem. 2008. PMID: 18691038 Review.
Cited by
-
Intrinsic hyporesponsiveness of invariant natural killer T cells precedes the onset of lupus.Clin Exp Immunol. 2013 Jul;173(1):18-27. doi: 10.1111/cei.12079. Clin Exp Immunol. 2013. PMID: 23607366 Free PMC article.
-
Immunoporosis: A New Role for Invariant Natural Killer T (NKT) Cells Through Overexpression of Nuclear Factor-κB Ligand (RANKL).Med Sci Monit. 2019 Mar 23;25:2151-2158. doi: 10.12659/MSM.912119. Med Sci Monit. 2019. PMID: 30903656 Free PMC article.
-
Therapeutic Potential of Invariant Natural Killer T Cells in Autoimmunity.Front Immunol. 2018 Mar 13;9:519. doi: 10.3389/fimmu.2018.00519. eCollection 2018. Front Immunol. 2018. PMID: 29593743 Free PMC article. Review.
-
Cell mediators of autoimmune hepatitis and their therapeutic implications.Dig Dis Sci. 2015 Jun;60(6):1528-42. doi: 10.1007/s10620-014-3473-z. Epub 2014 Dec 9. Dig Dis Sci. 2015. PMID: 25487192 Review.
-
Unconventional T cells and kidney disease.Nat Rev Nephrol. 2021 Dec;17(12):795-813. doi: 10.1038/s41581-021-00466-8. Epub 2021 Aug 26. Nat Rev Nephrol. 2021. PMID: 34446934 Review.
References
-
- Bendelac A. CD1: presenting unusual antigens to unusual T lymphocytes. Science. 1995;269:185–186. - PubMed
-
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–237. - PubMed
-
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

