Dihydroartemisinin-piperaquine versus chloroquine in the treatment of Plasmodium vivax malaria in Thailand: a randomized controlled trial
- PMID: 22002979
- PMCID: PMC3193831
- DOI: 10.1093/cid/cir631
Dihydroartemisinin-piperaquine versus chloroquine in the treatment of Plasmodium vivax malaria in Thailand: a randomized controlled trial
Abstract
Background: Chloroquine (CQ) remains the treatment of choice for Plasmodium vivax malaria. Initially confined to parts of Indonesia and Papua, resistance of P. vivax to CQ seems to be spreading, and alternative treatments are required.
Methods: We conducted a randomized controlled study to compare the efficacy and the tolerability of CQ and dihydroartemisinin-piperaquine (DP) in 500 adults and children with acute vivax malaria on the Northwestern border of Thailand.
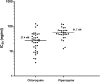
Results: Both drugs were well tolerated. Fever and parasite clearance times were slower in the CQ than in the DP group (P < .001). By day 28, recurrent infections had emerged in 18 of 207 CQ recipients compared with 5 of 230 treated with DP (relative risk, 4.0; 95% confidence interval [CI], 1.51-10.58; P = .0046). The cumulative risk of recurrence with P. vivax at 9 weeks was 79.1% (95% CI, 73.5%-84.8%) in patients treated with CQ compared with 54.9% (95% CI, 48.2%-61.6%) in those receiving DP (hazard ratio [HR], 2.27; 95% CI, 1.8-2.9; P < .001). Children <5 years old were at greater risk of recurrent P. vivax infection (74.4%; 95% CI, 63.2%-85.6%) than older patients (55.3% [95% CI, 50.2%-60.4%]; HR, 1.58 [95% CI, 1.1-2.2]; P = .005). In vitro susceptibility testing showed that 13% of the tested isolates had a CQ median inhibitory concentration >100 nmol/L, suggesting reduced susceptibility.
Conclusions: The efficacy of CQ in the treatment of P. vivax infections is declining on the Thai-Myanmar border. DP is an effective alternative treatment.
Figures