Mortality trends in the US Perinatal AIDS Collaborative Transmission Study (1986-2004)
- PMID: 22002982
- PMCID: PMC3202314
- DOI: 10.1093/cid/cir641
Mortality trends in the US Perinatal AIDS Collaborative Transmission Study (1986-2004)
Abstract
Background: Highly active antiretroviral therapy (HAART) has improved human immunodeficiency virus (HIV)-associated morbidity and mortality. The bimodal mortality distribution in HIV-infected children makes it important to evaluate temporal effects of HAART among a birth cohort with long-term, prospective follow-up.
Methods: Perinatal AIDS Collaborative Transmission Study (PACTS)/PACTS-HIV Follow-up of Perinatally Exposed Children (HOPE) study was a Centers for Disease Control and Prevention-sponsored multicenter, prospective birth cohort study of HIV-exposed uninfected and infected infants from 1985 until 2004. Mortality was evaluated for the no/monotherapy, mono-/dual-therapy, and HAART eras, that is, 1 January 1986 through 31 December 1990, from 1 January 1991 through 31 December 1996, and 1 January 1997 through 31 December 2004.
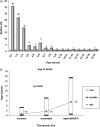
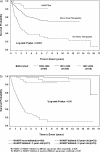
Results: Among 364 HIV-infected children, 56% were female and 69% black non-Hispanic. Of 98 deaths, 79 (81%) and 61 (62%) occurred in children ≤3 and ≤2 years old, respectively. The median age at death increased significantly across the eras (P < .0001). The average annual mortality rates were 18 (95% confidence interval [CI], 11.6-26.8), 6.9 (95% CI, 5.4-8.8), and 0.8 (95% CI, 0.4-1.5) events per 100 person-years for the no/monotherapy, mono-/dual-therapy and HAART eras, respectively. The corresponding 6-year survival rates for children born in these eras were 57%, 76%, and 91%, respectively (P < .0001). Among children who received HAART in the first 6 months of age, the probability of 6-year survival was 94%. Ten-year survival rates for HAART and non-HAART recipients were 94% and 45% (P < .05). HAART-associated reductions in mortality remained significant after adjustment for confounders (hazard ratio, 0.3; 95% CI, .08-.76). Opportunistic infections (OIs) caused 31.8%, 16.9%, and 9.1% of deaths across the respective eras (P = .051).
Conclusions: A significant decrease in annual mortality and a prolongation in survival were seen in this US perinatal cohort of HIV-infected children. Temporal decreases in OI-associated mortality resulted in relative proportional increases of non-OI-associated deaths.
Figures

Comment in
-
Highly active antiretroviral treatment and children.Clin Infect Dis. 2011 Nov;53(10):1035-6. doi: 10.1093/cid/cir638. Clin Infect Dis. 2011. PMID: 22002983 No abstract available.
References
-
- Detels R, Tarwater P, Phair JP, Margolick J, Riddler SA, Munoz A. Effectiveness of potent antiretroviral therapies on the incidence of opportunistic infections before and after AIDS diagnosis. AIDS. 2001;15:347–55. - PubMed
-
- Mocroft A, Katlama C, Johnson AM, et al. AIDS across Europe, 1994-98: the EuroSIDA study. Lancet. 2000;356:291–6. - PubMed
-
- Moore RD, Chaisson RE. Natural history of HIV infection in the era of combination antiretroviral therapy. AIDS. 1999;13:1933–42. - PubMed
-
- Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. - PubMed
-
- Vittinghoff E, Scheer S, O'Malley P, Colfax G, Holmberg SD, Buchbinder SP. Combination antiretroviral therapy and recent declines in AIDS incidence and mortality. J Infect Dis. 1999;179:717–20. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

