Carboxylases in natural and synthetic microbial pathways
- PMID: 22003013
- PMCID: PMC3233076
- DOI: 10.1128/AEM.05702-11
Carboxylases in natural and synthetic microbial pathways
Abstract
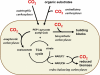
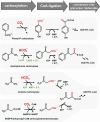
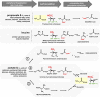
Carboxylases are among the most important enzymes in the biosphere, because they catalyze a key reaction in the global carbon cycle: the fixation of inorganic carbon (CO₂). This minireview discusses the physiological roles of carboxylases in different microbial pathways that range from autotrophy, carbon assimilation, and anaplerosis to biosynthetic and redox-balancing functions. In addition, the current and possible future uses of carboxylation reactions in synthetic biology are discussed. Such uses include the possible transformation of the greenhouse gas carbon dioxide into value-added compounds and the production of novel antibiotics.
Figures

References
-
- Abu Laban N., Selesi D., Rattei T., Tischler P., Meckenstock R. U. 2010. Identification of enzymes involved in anaerobic benzene degradation by a strictly anaerobic iron-reducing enrichment culture. Environ. Microbiol. 12:2783–2796 - PubMed
-
- Adams M. W. W., Kletzin A. 1996. Oxidoreductase-type enzymes and redox proteins involved in fermentative metabolisms of hyperthermophilic archaea. Adv. Protein Chem. 48:101–180 - PubMed
-
- Advanced Research Projects Agency 5 May 2011, accession date ARPA-E electrofuels project summary. U.S. Department of Energy, Washington, DC: http://arpa-e.energy.gov/LinkClick.aspx?fileticket=TRv8LDEgnhw%3D&tabid=82
-
- Anthony C. 1982. Methylotrophic bacteria, p. 1–40 In The biochemistry of methylotrophs. Academic Press, London, United Kingdom
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

