Novel yeast bioassay for high-throughput screening of matrix metalloproteinase inhibitors
- PMID: 22003025
- PMCID: PMC3233093
- DOI: 10.1128/AEM.06111-11
Novel yeast bioassay for high-throughput screening of matrix metalloproteinase inhibitors
Abstract
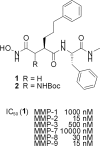
Diverse malfunctions in the expression and regulation of matrix metalloproteinases (MMPs) are often the cause of severe human diseases, bringing the identification of specific MMP inhibitors into major focus, particularly in anticancer treatment. Here, we describe a novel bioassay based on recombinant yeast cells (Pichia pastoris) that express, deliver, and incorporate biologically active human MMP-2 and MMP-9 at the yeast cell surface. Using Sed1p for cell wall targeting and covalent anchorage, a highly efficient bioassay was established that allows high-throughput screening and subsequent validation of novel MMP inhibitors as potential anticancer drugs. In addition, we developed a straightforward synthesis of a new aspartate-derived MMP inhibitor active in the nM range and bearing an amino functionality that should allow the introduction of a wide range of side chains to modify the properties of these compounds.
Figures


Similar articles
-
A new screening method based on yeast-expressed human dipeptidyl peptidase IV and discovery of novel inhibitors.Biotechnol Lett. 2009 Jul;31(7):979-84. doi: 10.1007/s10529-009-9963-y. Epub 2009 Mar 8. Biotechnol Lett. 2009. PMID: 19267232
-
Development and Application of the Metalloprotease Activity Multiplexed Bead-Based Immunoassay (MAMBI).Biochemistry. 2019 Sep 24;58(38):3938-3942. doi: 10.1021/acs.biochem.9b00584. Epub 2019 Sep 9. Biochemistry. 2019. PMID: 31474112
-
Characterization of C-terminally truncated human tissue inhibitor of metalloproteinases-4 expressed in Pichia pastoris.Biol Chem. 2001 Jun;382(6):987-91. doi: 10.1515/BC.2001.124. Biol Chem. 2001. PMID: 11501766
-
Matrix Metalloproteinases (MMPs) in Targeted Drug Delivery: Synthesis of a Potent and Highly Selective Inhibitor against Matrix Metalloproteinase- 7.Curr Top Med Chem. 2020;20(27):2459-2471. doi: 10.2174/1568026620666200722104928. Curr Top Med Chem. 2020. PMID: 32703131 Review.
-
Recent opportunities in matrix metalloproteinase inhibitor drug design for cancer.Expert Opin Drug Discov. 2018 Jan;13(1):75-87. doi: 10.1080/17460441.2018.1398732. Epub 2017 Oct 31. Expert Opin Drug Discov. 2018. PMID: 29088927 Review.
Cited by
-
Characterization and regulation of MT1-MMP cell surface-associated activity.Chem Biol Drug Des. 2019 Jun;93(6):1251-1264. doi: 10.1111/cbdd.13450. Epub 2018 Dec 19. Chem Biol Drug Des. 2019. PMID: 30480376 Free PMC article.
-
Yeast-Based Biosensors: Current Applications and New Developments.Biosensors (Basel). 2020 May 13;10(5):51. doi: 10.3390/bios10050051. Biosensors (Basel). 2020. PMID: 32413968 Free PMC article. Review.
References
-
- Babine R. E., Bender S. L. 1997. Molecular recognition of protein-ligand complexes: applications to drug design. Chem. Rev. 97:1359–1472 - PubMed
-
- Corbitt C. A., Lin J., Lindsey M. L. 2007. Mechanisms to inhibit matrix metalloproteinase activity: where are we in the development of clinically relevant inhibitors? Recent Pat. Anticancer Drug Discov. 2:135–142 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

